How would you know if an element is an isotope?
1 Answer
An isotope is just a form that an element can exist as. Recall that elements are made distinct from one another by their proton numbers only; provided several nuclei contain the same number of protons, those nuclei will correspond to a certain element regardless of how many neutrons they contain.
Different isotopes of an element can be identified via mass spectrometry: one instance of this process is known as time of flight mass spectrometry ( TOFMS). This works by ionising a gaseous sample of whatever it is you're trying to study, be it an atom or molecule. Let's work with the example of chlorine: when gaseous chlorine molecules are ionised, they fragment to give 1 chloride ion and 1 chlorine atom.
The chloride ions that result from this process are accelerated so that each ion has the same kinetic energy: because of this, various chlorine ions will move at different speeds through the flight tube / drift region of the spectrometer due to them having different masses. More massive ions will move more slowly through the spectrometer, because mass is inversely proportional to speed.
When the ions hit the ion detector at the back of the mass spectrometer, they cause an electric current to be generated. By linking the spectrometer up to a computer, the information can be interpreted and a graph produced. This graph will show us several peaks: one for each significant isotope of the element chlorine. The mass spectrometry graph of chlorine is shown below.
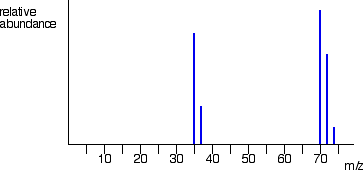
You will notice that there are peaks at
You will also see peaks at
- chlorine-35 + chlorine-35
# = 70# - chlorine-35 + chlorine-37
# = 72# - chlorine-37 + chlorine-37
# = 74#

