Can someone help with a and c?
1 Answer
Here's what I get.
Explanation:
(a) Separating the mixture
One of the compounds is a carboxylic acid; the other is an amine.
We can use their acidic and basic properties to separate and purify them.
For example, an aromatic acid is often insoluble in water but soluble in ether. Its carboxylate salt is soluble in water but insoluble in ether.
Similarly, an aromatic amine may be insoluble in water but soluble in ether. Its ammonium salt is soluble in water but not in ether.
Scheme 1. Separating the amine first
Here's a flowchart that I would use to develop a separation procedure.
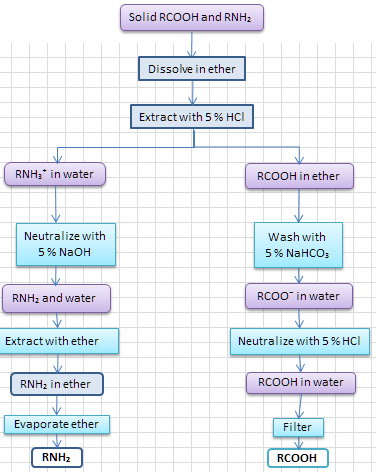
- Dissolve the mixture in diethyl ether.
- Transfer to a separatory funnel. Then add dil.
#"HCl"# and shake well to extract the amine salt. -
Draw off the aqueous layer (Solution A) and set aside the ether layer (Solution B) for later.
-
Neutralize Solution A with dil.
#"NaOH"# and extract with ether. - Dry the ether solution over anhydrous
#"MgSO"_4# . -
Filter off the
#"MgSO"_4# , evaporate the ether and purify the amine by crystallization from a suitable solvent. -
Wash Solution A with dil.
#"NaHCO"_3# . - Neutralize the aqueous layer with dil.
#"HCl"# - Filter off the precipitated
#"RCOOH"# , dry it in the air and recrystallize from a suitable solvent (aqueous ethanol?).
Scheme 2. Separating the acid first
You could also separate the acid first. Your flowchart might look like this.
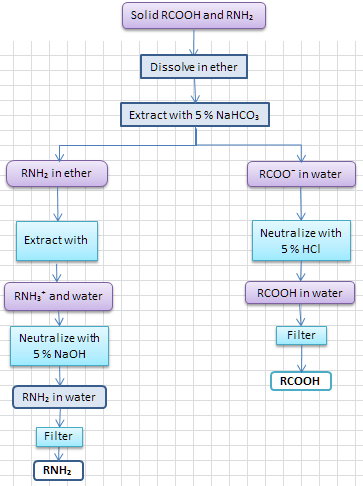
(c) Purification by recrystallization
Here's one way you might do it.
- Place the solid in an Erlenmeyer flask.
- Add a few millilitres of ethanol and warm the flask until all the solid dissolves.
- Remove the flask from the heat and slowly add water until crystals just start to form.
- Chill the flask and contents in an ice-water bath.
- Collect the crystals using suction filtration and allow them to dry.

