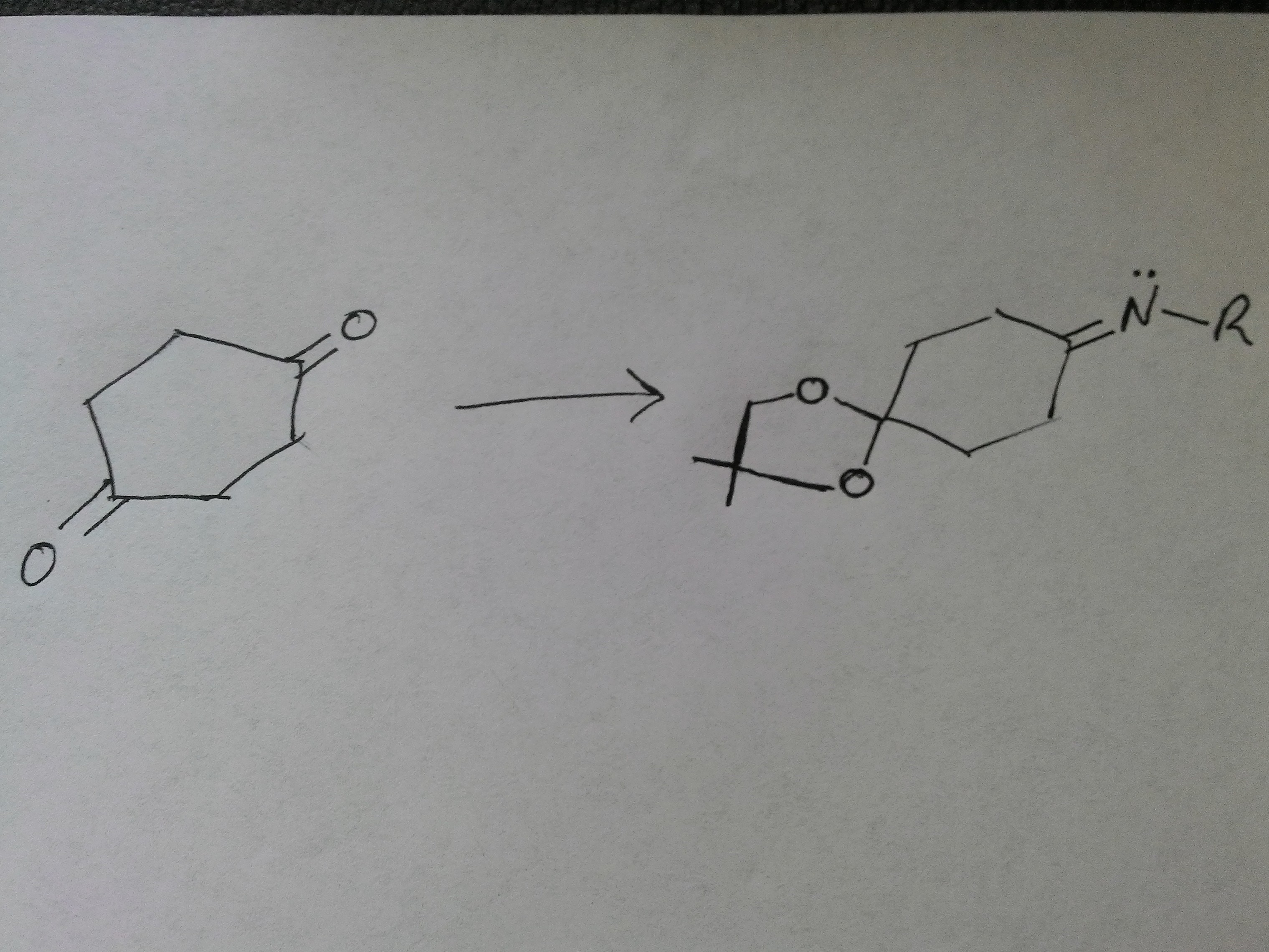
I am looking for a nudge in the right direction (not so much an answer) as to how to react 1,4-cyclohexanedione to produce the product as seen in the attached image. I am stuck due to the symmetry of the starting material. Am I overthinking it? Thank you
1 Answer
Mar 18, 2018
I think you could be overthinking it mate....
Explanation:
You got TWO carbonyls in the SYMMETRIC starting material. You protect ONE with ONE EQUIV of the dialcohol (the which I think you have represented improperly). With that accomplished you react the OTHER carbonyl with an amine to form the imine. I would post some pictures, but I ain't got ChemDraw available....

