I have read that when metal like Na, K, Be put into water , heat is evolved. Can we call this heat of hydration? Please let me know what is heat of hydration actually?
1 Answer
That is an exothermic heat of reaction, not hydration. You could call that
Heat of hydration is generally not able to be isolated.
When dissolving an ionic compound, the enthalpy change for an exothermic dissolution is:
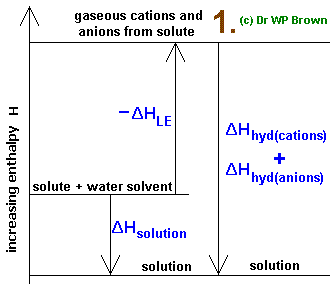
#DeltaH_"solution" = " "overbrace(DeltaH_"solute")^((+)) + overbrace(DeltaH_"solvent")^((+)) +" " overbrace(DeltaH_"mix")^((-))#
#= -overbrace(DeltaH_"LE")^((-)) + " "" "" "overbrace(DeltaH_"hyd")^("unclear sign")# where:
#DeltaH_"solute"# is the enthalpy change for breaking apart solute particles from interacting with themselves.This is always positive.
#DeltaH_"solvent"# is the enthalpy change for breaking apart solvent particles from interacting with themselves.This is always positive.
#DeltaH_"mix"# is the enthalpy change for combining the solute and solvent particles so they can interact with each other.This is always negative.
#DeltaH_"LE" -= -DeltaH_"solute"# is the lattice energy, i.e. the energy needed to form a solid lattice structure from the uncombined gaseous ions.This is always negative.
#DeltaH_"hyd"# is the enthalpy change of hydration, i.e. the enthalpy change due to separating the solvent particles from themselves (#DeltaH_"solvent"# ) plus making them surround the solute particles (#DeltaH_"mix"# ).This has an ambiguous sign (depending on the relative sizes of
#DeltaH_(solvent)# and#DeltaH_(mix)# ).
When you put REACTIVE metals into water, the only thing that changes is that
#DeltaH_"lattice"# doesn't apply since the metal is a network solid of cations in a sea of electrons, with no anions to speak of.#DeltaH_"hyd"# is even harder to track, because upon surrounding the solute metal particles, oxidation of the metal tends to occur.
For instance,
#2"Na"(s) + 2"H"_2"O"(l) -> 2"NaOH"(aq) + "H"_2(g)#
So while hydration should still occur, it is perhaps more short-lived if a reaction occurs.

