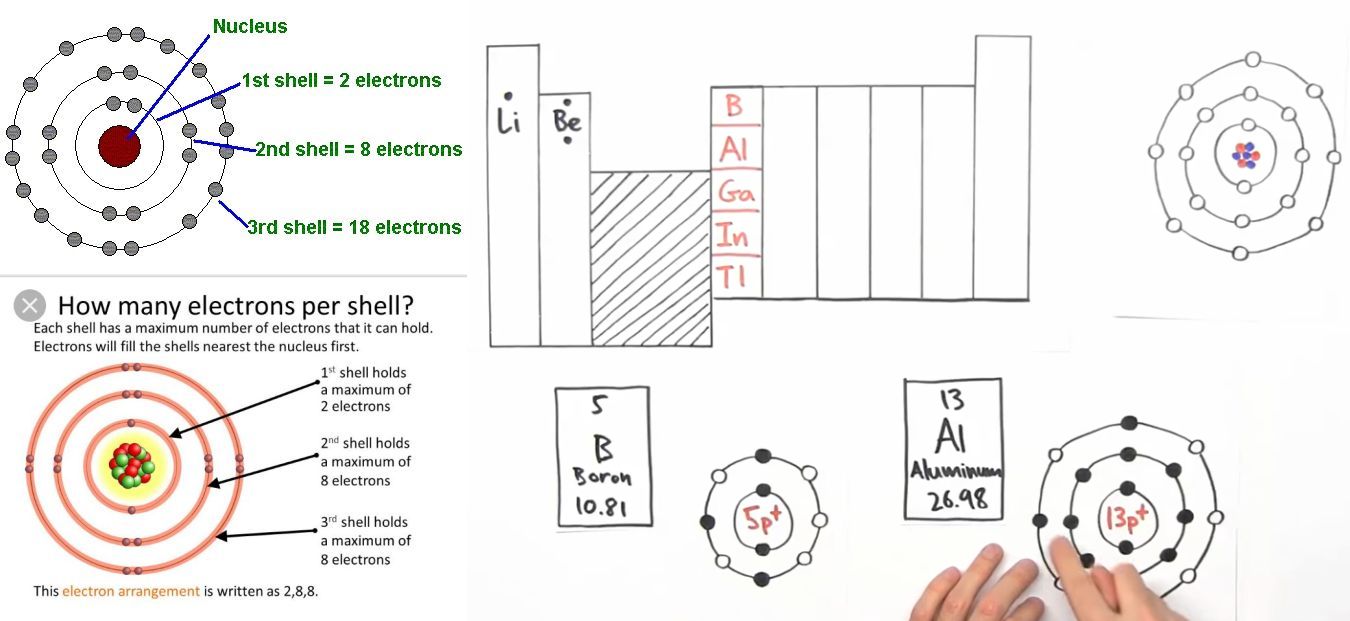
Maximum number of electrons a shell can accommodate is "2n"^2.
Third shell contains maximum 2 × (3)^2 = 18 electrons. The one given in the image is incorrect.
underline(bb"Shell (n)" color(white)(.......) bb("Maximum electrons" ("2n"^2)))
color(white)(...)1 color(white)(..................................) 2
color(white)(...) 2 color(white)(..................................) 8
color(white)(...) 3 color(white)(.................................) 18
color(white)(...) 4 color(white)(................................) 32
What you need to know is that when a shell is being filled there is no necessary requirement that inner shells should be completely filled.
Electrons are filled based on Aufbau principle. For e.g. when 3^"rd" shell (which can accommodate maximum 18 electrons) is filled with 8 electrons we start filling 4^"th" shell (which can accommodate maximum 32 electrons). When 4^"th" shell is filled with 2 electrons we then start filling electrons in 3^"rd" shell until it is completely filled. Note here that we have filled 2 electrons in 4^"th" shell before completely filling 3^"rd" shell.
For first 18 elements of periodic table electronic configuration is in the order 2,8,8. For these elements when electron is filled in n^"th" shell (n - 1)^"th" shell should be completely filled.
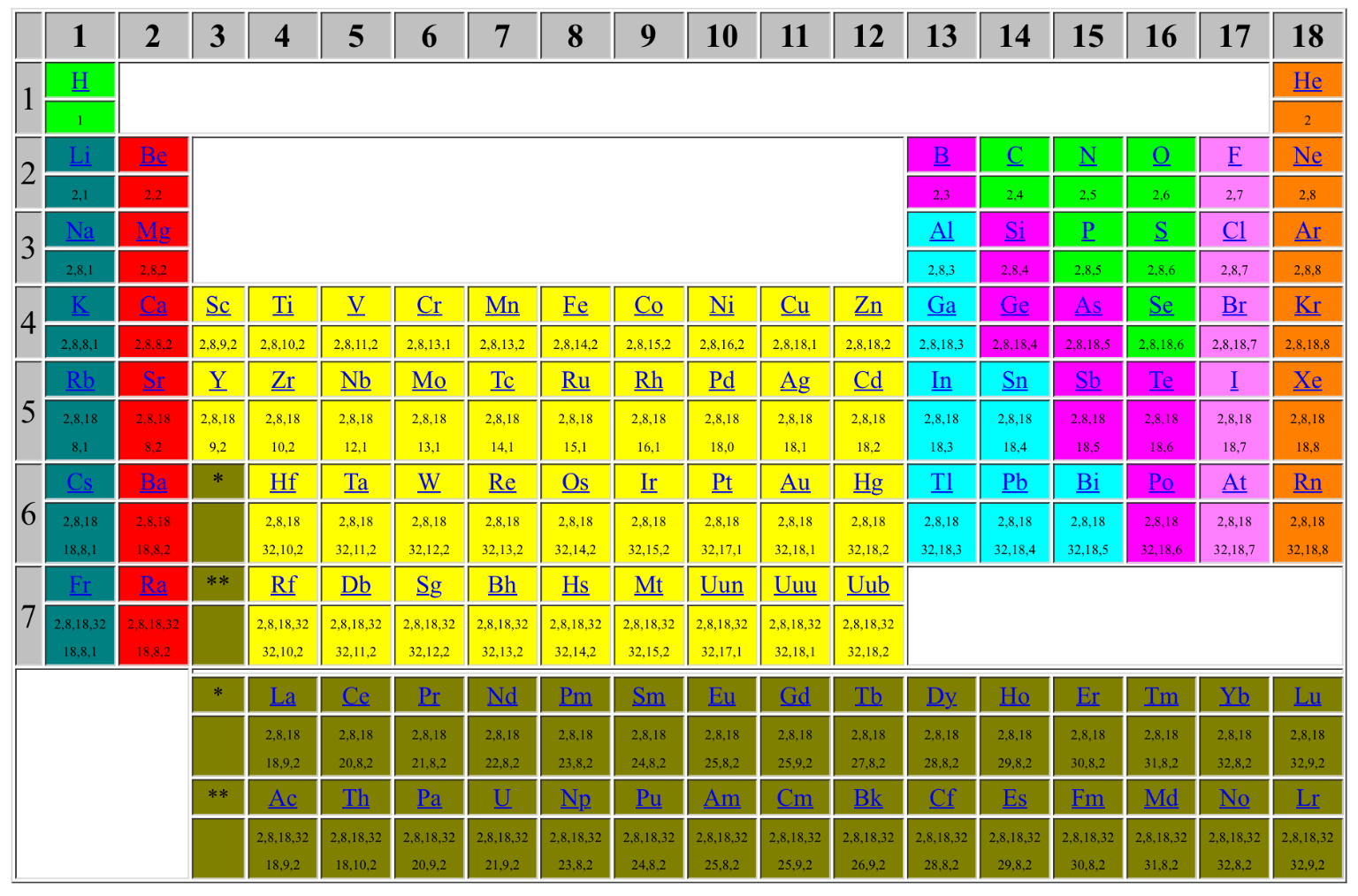
underline("Additional:") 2,8,8,18,18,32 is difference in number of protons (or electrons) when we move from top to to bottom of same group. For e.g. differences in number of electrons of "Na" and "Li", "K" and "Na", "Rb" and "K", "Cs" and "Rb", "Fr" and "Cs" is 8,8,18,18,32 respectively.