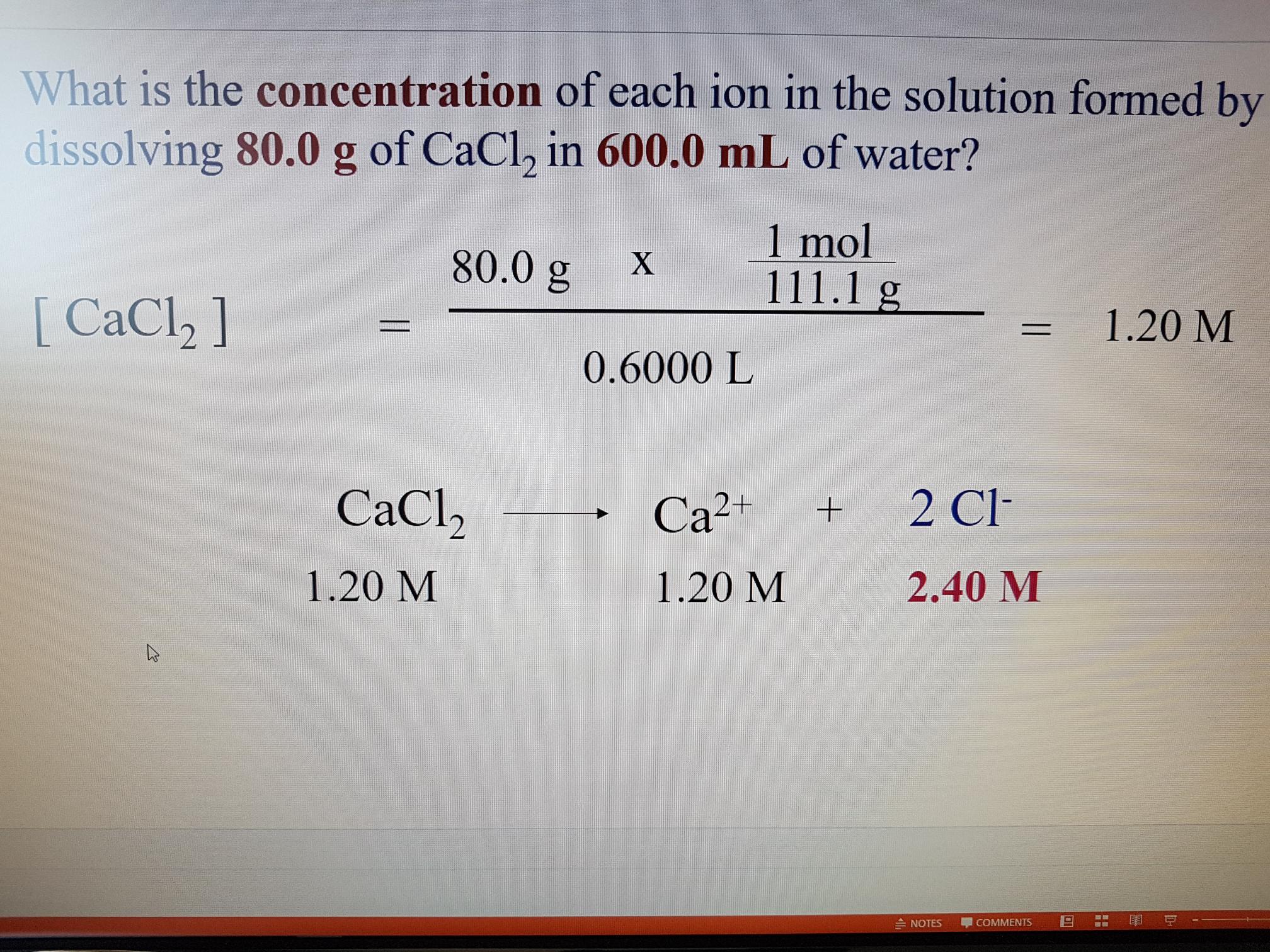
I understand the top half, and got the same answer (1.20M) but I don't know how they figured out the molarity for Ca2+ and 2Cl- Can someone help clarify this?
1 Answer
May 16, 2018
Consider the speciation that calcium chloride undergoes in aqueous solution....
Explanation:
We represent the chemical reaction as....
And so ONE equiv of SOLID salt, undergoes reaction to GIVE ONE EQUIV of metal dication and TWO EQUIV of CHLORIDE ANION....and so each equiv of calcium chloride salt gives THREE equiv of ions....
And so while
Am I reading you right? If I am barking up the wrong tree again, I apologize...

