If 1.000g of tin metal reacts with 0.640g of fluorine gas, what is the empirical formula of the product?
1 Answer
Explanation:
The empirical formula of a given compound tells you the smallest whole number ratio in which its constituent elements combine to form said compound.
In your case, an unknown compound is said to contain tin,
It's important to realize that fluorine gas exists as diatomic molecules, and so its molar mass will be twice as big as the molar mass of fluorine,
Grab a periodic table and look for tin and fluorine. Their molar masses are
#M_("M Sn") = "118.72 g mol"^(-1)#
#M_("M F") = "18.998 g mol"^(-1)#
The molar mass of fluorine gas will thus be
#M_("M F"_ 2) = 2 xx M_("M F")#
#M_("M F"_ 2) = 2 xx "18.998 g mol"^(-1) = "37.996 g mol"^(-1)#
This means that your sample of fluorine gas contained
#0.640 color(red)(cancel(color(black)("g"))) * "1 mole F"_2/(37.996color(red)(cancel(color(black)("g")))) = "0.016844 moles F"_2#
Since every molecule of
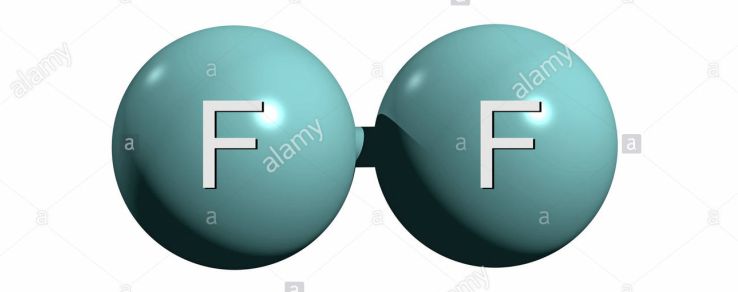
you know that your unknown compound will contain
#0.016844 color(red)(cancel(color(black)("moles F"_2))) * "2 moles F"/(1color(red)(cancel(color(black)("mole F"_2)))) = "0.033688 moles F"#
The sample will also contain
#1.000 color(red)(cancel(color(black)("g"))) * "1 mole Sn"/(118.72 color(red)(cancel(color(black)("g")))) = "0.0084232 moles Sn"#
To find the mole ratio that exists between the two elements in the unknown compound, divide both values by the smallest one
#"For Sn: " (0.0084232 color(red)(cancel(color(black)("moles"))))/(0.0084232color(red)(cancel(color(black)("moles")))) = 1#
#"For F: " (0.033688color(red)(cancel(color(black)("moles"))))/(0.0084232color(red)(cancel(color(black)("moles")))) = 3.999 ~~ 4#
Since
#"Sn"_1"F"_4 implies color(green)(|bar(ul(color(white)(a/a)color(black)("SnF"_4)color(white)(a/a)|)))#

