If 1M of HCl is placed with 1L of water, how would the amount of H3O+ compare to that of a solution made from 1M of acetic acid placed in 1L of water?
1 Answer
The solution with
Explanation:
First, let's establish that
They're both acids, which means that, according to the Bronsted-Lowry definition, both of them will donate
These
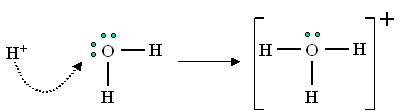
A strong acid is one that almost completely dissociates to donate
So, if the same amount of a strong acid (

