If #50.0# milliliters of 3.0 M #"H"_3"PO"_4# completely neutralized #150.0# milliliters of #"Mg"("OH")_2#, what was the molarity of the #"Mg"("OH")_2# solution?
1 Answer
This is an exceptionally poor question....
Explanation:
Phosphoric acid acts as a DIACID under normal conditions...and so we write the stoichiometric equation...
With respect to phosphoric acid, we got a molar quantity of...
And thus we SUPPOSE it to have neutralized
Magnesium hydroxide is not so soluble in aqueous solution. The person who set this question was not a chemist. A solution of
In the following graph, phosphoric acid is titrated with sodium hydroxide; both solutions are
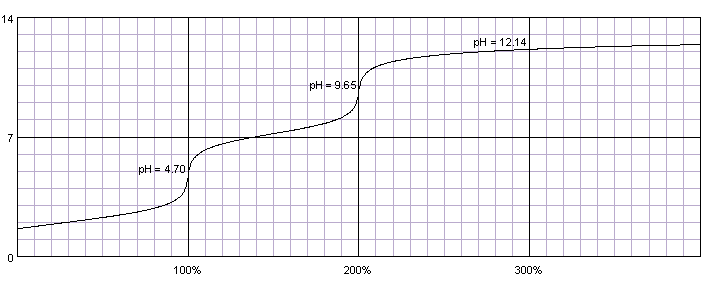
The two equivalence points are clear....

