If an element is located on the far right side of the periodic table, what would one of its physical properties probably be?
1 Answer
Jul 11, 2018
You have not specified an orientation....which of course is a fundamental principle of chemistry....
Explanation:
But as we FACE the Periodic Table, the elements on OUR right hand side are the Noble Gases...:
And these beasts are supremely UNREACTIVE, and undergo reactions with ONLY the most powerful oxidants, i.e. with difluorine, and dioxygen... Given their full valence electronic shell, the ELEMENTS are encountered as room temperature gases.
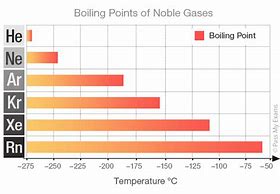
Conveniently the graph has units of

