If each atomic orbital can accommodate maximum 5 electrons, then the maximum number of elements possible in #8^"th"# period would be?
(1) 80
(2) 64
(3) 105
(4) 125
(1) 80
(2) 64
(3) 105
(4) 125
1 Answer
The maximum number of elements possible in the 8th period would be (4) 125.
Explanation:
We must derive the electron configuration of the 8th period, assuming that the orbitals follow the (
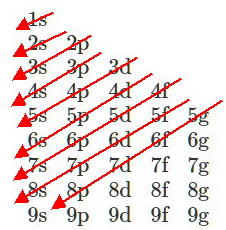
The order of orbitals in the eighth period would be
#"8s"color(white)(l)"5g"color(white)(l)"6f"color(white)(l)"7d"color(white)(l)"8p"#
The number of electrons in each orbital is
#ulbb("Orbital"color(white)(m)"Electrons"color(white)(m))#
#color(white)(mll)"8s"color(white)(mmll)1×5 =color(white)(ll)5#
#color(white)(mll)"8p"color(white)(mml)3×5 =color(white)(l) 15#
#color(white)(mll)"7d"color(white)(mml)5 × 5 =color(white)(l) 25#
#color(white)(mll)"6f"color(white)(mmll)7 × 5 =color(white)(l) 35#
#ul(color(white)(mll)"5g"color(white)(mml)9 ×5 =color(white)(l) 45)#
#color(white)(mmmmm)"Total" = 125#
Thus, there would be 125 elements in the eighth period.

