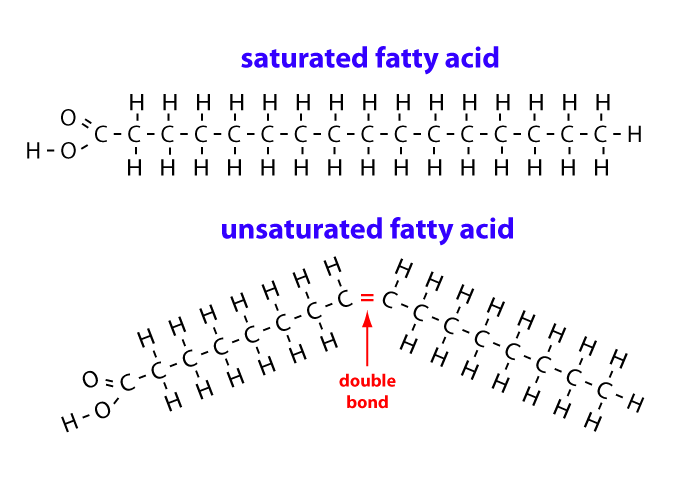
If you were comparing a saturated fatty acid with an unsaturated fatty acid and both were the same length, how could you discriminate between the two?
1 Answer
The unsaturated fatty acid would have at least one pair of double bonded carbon atoms.
Explanation:
In fatty acids, saturation has to do with hydrogen atoms in the hydrocarbon chain, which is determined by the presence or absence of double-bonded carbon atoms.
A saturated fatty acid has no double-bonded carbon atoms, so it is saturated with hydrogen atoms. Every carbon atom in the hydrocarbon chain is bonded to two hydrogen atoms, which is the maximum.
An unsaturated fatty acid has at least one double-bonded pair of carbon atoms in the hydrocarbon chain. These two carbon atoms can only bond with one hydrogen atom each, so the fatty acid chain has fewer hydrogen atoms, which makes it unsaturated.
Both of the fatty acids below have 15 carbon atoms in their hydrocarbon chains. The saturated fatty acid has no double-bonded carbon atoms, so it is saturated with hydrogen atoms. The unsaturated fatty acid has one double-bonded pair of carbon atoms, and it has two fewer hydrogen atoms, which makes it unsaturated.