In 100 g of water at 0°C, table sugar has a solubility of 180 g. What will happen if you add 100 g of table sugar to 50 g of water at 0°C?
1 Answer
You will produce a saturated solution.
Explanation:
Sugar is said to have a solubility of
A saturated solution is a solution in which the rate at which solid particles are dissolved is equal to the rate at which dissolved solute particles reform the solid.
Simply put, a saturated solution is a solution that cannot dissolve more solute than what is already dissolved.
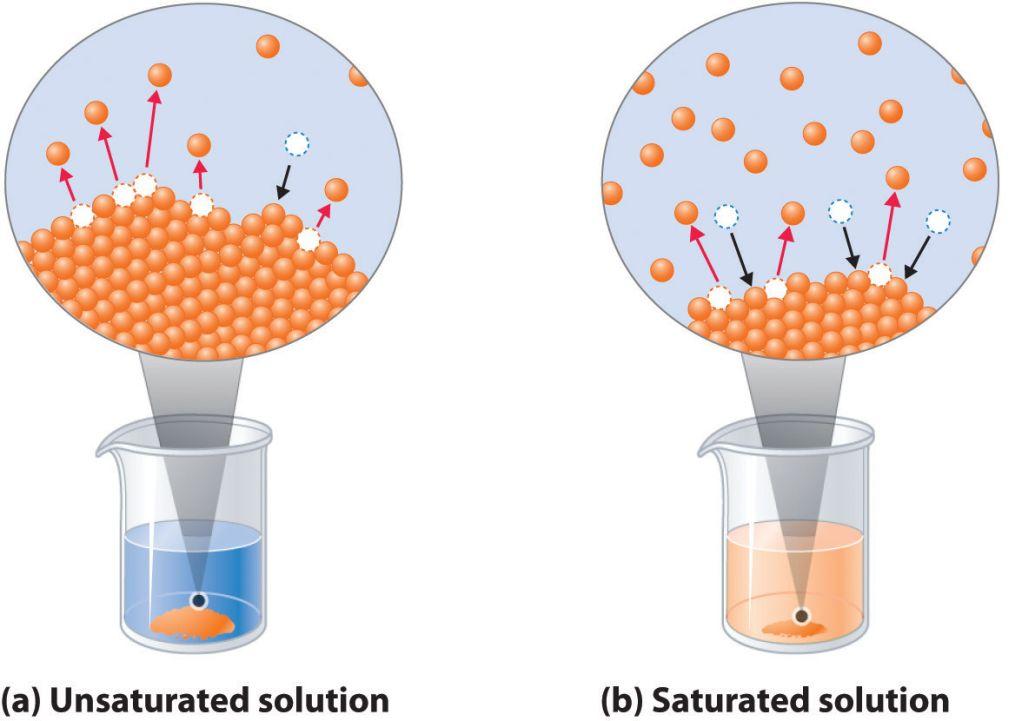
By comparison, in an unsaturated solution, the rate at which solid particles are dissolved exceeds the rate at which dissolved particles reform the solid, which means that the solution can dissolve more solute than what is already dissolved.
In your case, adding
#50 color(red)(cancel(color(black)("g water"))) * "180 g sugar"/(100color(red)(cancel(color(black)("g water")))) = "90 g sugar"#
This means that adding
Now, you want to add
More specifically, it will contain
#m_"undissolved sugar" = overbrace("100 g")^(color(purple)("amount added")) - overbrace("90 g")^(color(blue)("amount dissolved"))#
#m_"undissolved sugar" = "10 g"#
Therefore, adding

