In a closed can of soda, dissolved carbonic acid is in equilibrium with CO2 and H2O. When the can is opened CO2 is produced until the pop goes flat. How do these observations relate to equilibrium and Le Chatelier's principle?
1 Answer
Le Châtelier's Principle states that when a stress is applied to a system at equilibrium, the system will respond in such a way that it relieves the stress.
See What is Le Chatelier's principle?.
A can of soda is a closed system. The CO₂ in solution is in equilibrium with the CO₂ in the gas phase.
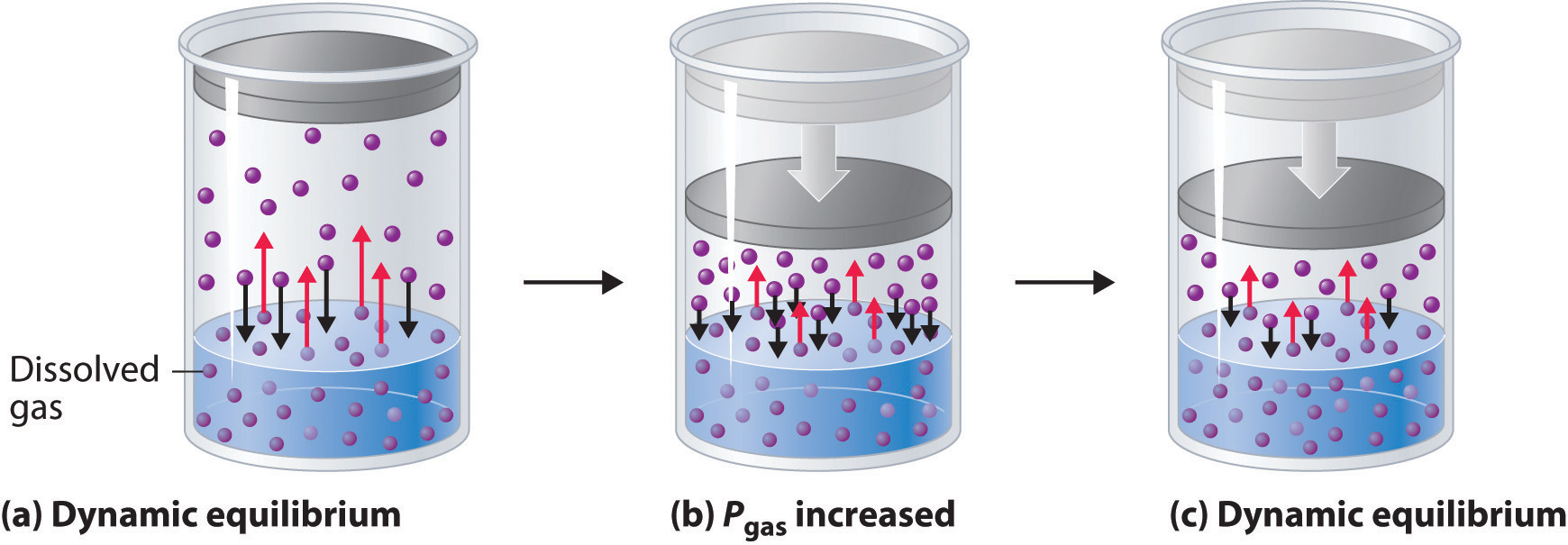
CO₂ molecules leave the solution at the same rate as they return.
This is a dynamic equilibrium, and it corresponds to a certain pressure of CO₂ in the container.
We represent the equilibrium as
CO₂(aq) ⇌ CO₂(g)
When you open the can, you no longer have a closed system. The CO₂ molecules are free to escape into the atmosphere.

This decreases the number of molecules returning to the solution.
The system is no longer at equilibrium. You are decreasing the concentration of the product.
According to Le Châtelier's Principle, the system responds by trying to replace the molecules that have escaped.
These molecules then escape into the atmosphere. The process continues until the pop goes flat.

