In a potential energy diagram for an endothermic reaction?
The answer options are:
1. Heat of reaction is negative.
2. Activation energy is positive.
3. The activation energy for the reverse reaction is smaller than that for the forward reaction.
4. Energy of products is lower than the energy of the reactants
The answer options are:
1. Heat of reaction is negative.
2. Activation energy is positive.
3. The activation energy for the reverse reaction is smaller than that for the forward reaction.
4. Energy of products is lower than the energy of the reactants
1 Answer
Mar 3, 2018
2. and 3. are true.
Explanation:
Here is a potential energy diagram for an endothermic reaction.
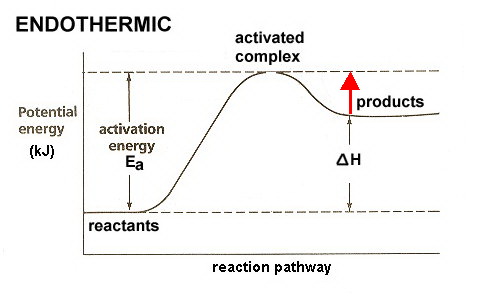
(Adapted from 1.bp.blogspot.com)
1. False
2. True
3. True
The red arrow in the diagram represents
4. False

