In #ddotCX_2#,both the electron get paired up in #sp^2# hybrid orbital.Thus,#ddotCX_2# exists in the form of singlet state.Why?{here #X# refers to halogen}
1 Answer
Because an even number of electrons are placed into a set of only singly-degenerate molecular orbitals. Thus, spin-pairing is going to happen for all the filled molecular orbitals, leading to a singlet state, i.e. diamagnetic molecule.
In the MO diagram below, the
With orbital potential energies from here (Appendix B.9), an MO diagram can be constructed for
We place the molecule on the
[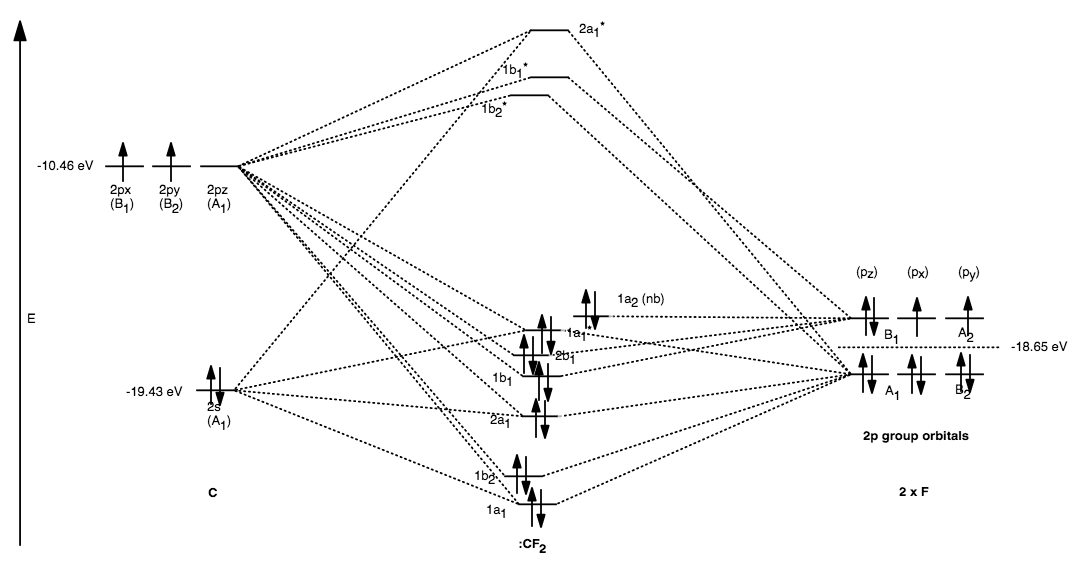
As you can see, there are only singly-degenerate molecular orbitals, which means there are no two molecular orbitals of identical energies. That arises when...
- Two different atomic orbitals interact, forming one bonding and one antibonding
#sigma# orbital, when no other atomic orbitals are the same energy within each atom.
[
- Three different atomic orbitals interact, forming one bonding, one nonbonding, and one antibonding
#sigma# orbital, when no other atomic orbitals are the same energy within each atom.
[
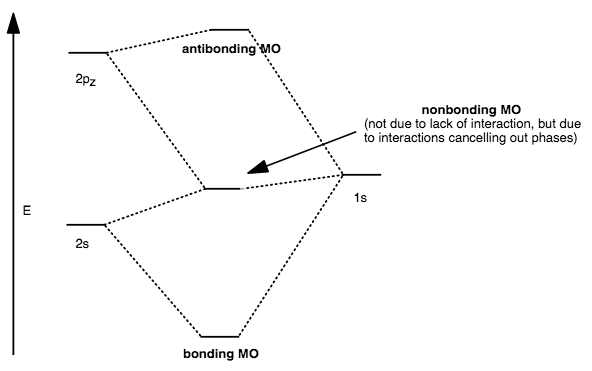
When the molecular orbitals are only singly-degenerate, no unpaired electrons can result when there are an even number of electrons coming from the atoms (
Since there are