In galvanic element Cr | Cr3+ ; Fe2+ | Fe in which direction will move the electrons?
1 Answer
May 17, 2018
Electrons will move from
Explanation:
The electrodes for this question would be
Here's a table of standard reduction potentials:
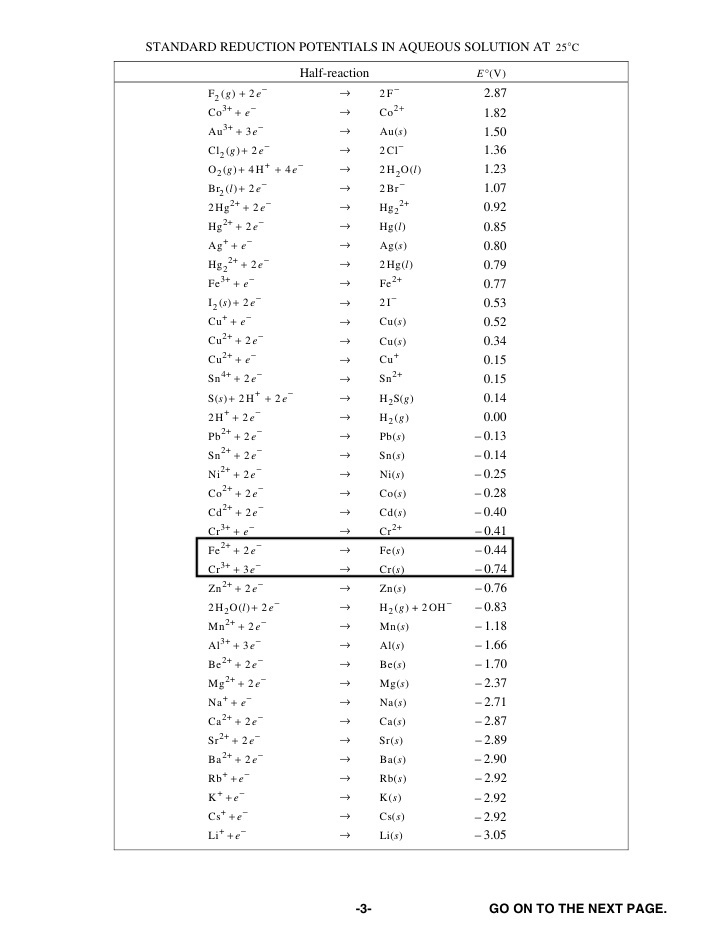
We can see that
So, in a galvanic cell with
Therefore, electrons will flow from

