In Period 2 of the Periodic Table, which Group contains the element with the highest first ionization energy?
1 Answer
Surely it is
Explanation:
We examine the rxn:
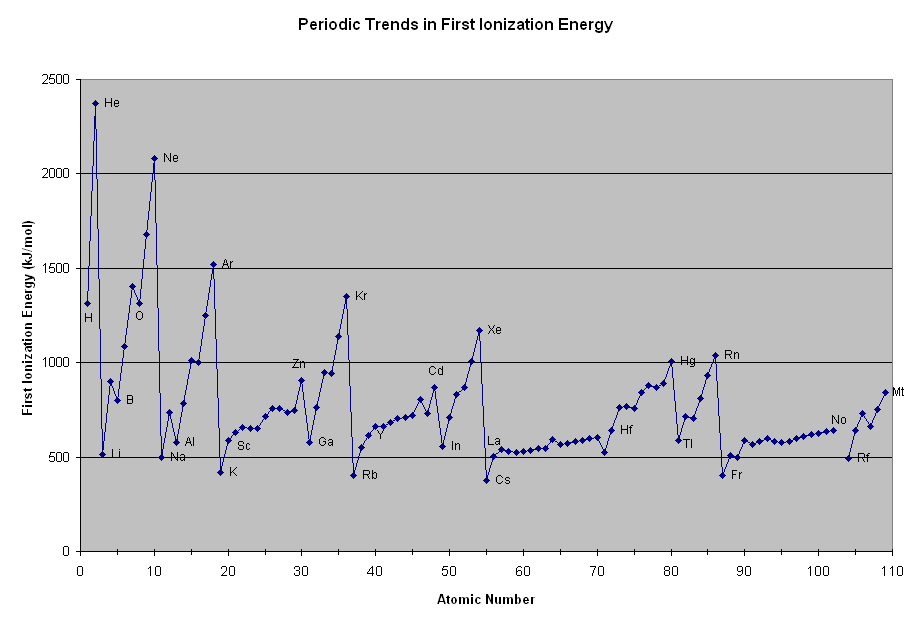
Ionization energy increases across a Period (from left to right as we face the Table). It decreases down a Group.
This is one of the most important manifestations of Periodicity. Incomplete electronic shells shield the nuclear charge very imperfectly, with the result that across the Period the atomic radius is known to decrease. When we reach the Noble Gases, however, the valence shell is full. And maximum attraction results between the nuclear charge and the valence electrons.
Anyway, as chemists, as physical scientists, we are obliged to look up the numbers, to see if the data agree with our notions of chemistry. And if they don't then our understanding is awry.
 www.angelo.edu/faculty/kboudrea/
www.angelo.edu/faculty/kboudrea/
And thus neon, with the greatest nuclear charge of the 2nd period, has the corresponding greatest ionization energy of the Period. Clearly, this property is shared by all the Noble Gases.

