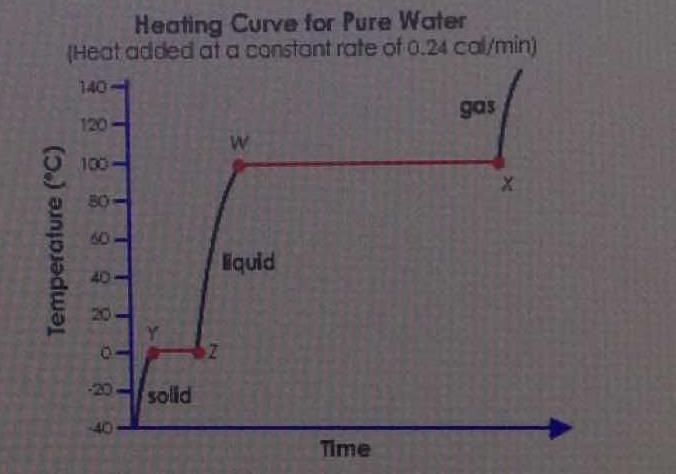
Because trying to get water to boil takes much longer than melting ice.
The #YZ# line is at a constant temperature. Specifically, at #0^@ "C"#. If you recognize that as the melting point of ice, we are evidently performing a melting phase transition by heating the ice over time along the #YZ# line.
The enthalpy of melting the ice, #DeltaH_(fus)#, is about #"6.02 kJ/mol"# at ordinary pressures. The enthalpy of vaporizing the water, #DeltaH_(vap)#, is about #"40.7 kJ/mol"#, which is larger.
The larger enthalpy indicates a larger amount of heat applied at constant pressure in order to accomplish the phase transition.
Since more heat was required to vaporize the water along the #WX# line, more time is thus required at some constant heating rate to vaporize the water along the #WX# line.