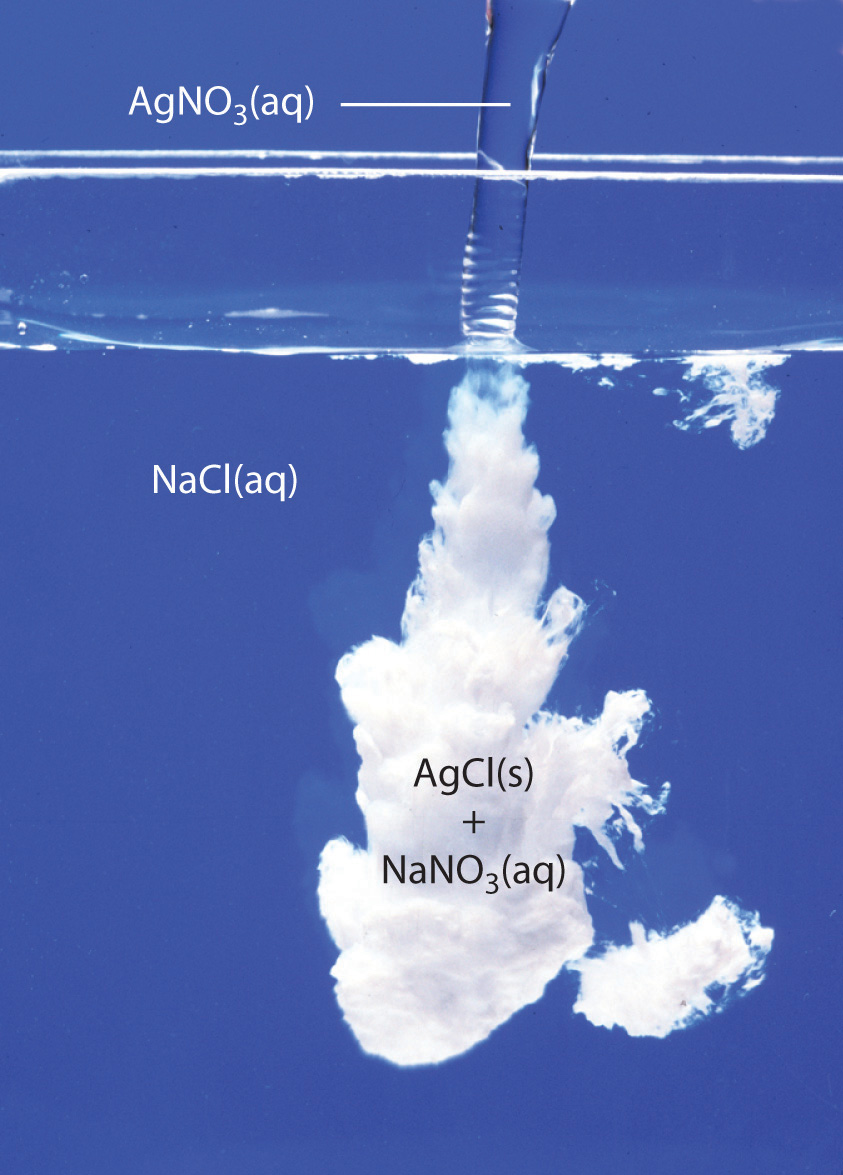
In the ionic equation for the reaction that occurs upon mixing aqueous solutions of #"NaCl"# and #"AgNO"_3#, what are the spectator ions?
1 Answer
Explanation:
When you mix aqueous solutions of sodium chloride and silver nitrate, a double replacement reaction that produces the insoluble silver chloride takes place.
The other product of the reaction is sodium nitrate, which is soluble in water. As a result, it will exist as ions in the solution.
This implies that the ions that do not combine to form the precipitate, i.e. the sodium cation
So, you know that the two reactants are soluble in water, so you can write them as
#"NaCl"_ ((aq)) -> "Na"_ ((aq))^(+) + "Cl"_ ((aq))^(-)#
#"AgNO"_ (3(aq)) -> "Ag"_ ((aq))^(+) + "NO"_ (3(aq))^(-)#
The complete ionic equation, which includes the spectator ions, will look like this
#"Na"_ ((aq))^(+) + "Cl"_ ((aq))^(-) + "Ag"_ ((aq))^(+) + "NO"_ (3(aq))^(-) -> "AgCl"_ ((s)) darr + "Na"_ ((aq))^(+) + "NO"_ (3(aq))^(-)#
If you eliminate the two spectator ions
#color(red)(cancel(color(black)("Na"_ ((aq))^(+)))) + "Cl"_ ((aq))^(-) + "Ag"_ ((aq))^(+) + color(red)(cancel(color(black)("NO"_ (3(aq))^(-)))) -> "AgCl"_ ((s)) darr + color(red)(cancel(color(black)("Na"_ ((aq))^(+)))) + color(red)(cancel(color(black)("NO"_ (3(aq))^(-))))#
you will end up with the net ionic equation
#"Ag"_ ((aq))^(+) + "Cl"_ ((aq))^(-) -> "AgCl"_ ((s)) darr#
It's worth mentioning that silver chloride is a white insoluble solid that will precipitate out of the solution.