In the Lewis structure of SO3. What is the formal charge of atom O?
1 Answer
Mar 21, 2018
This is an old chestnut....we gots 24 valence electrons to distribute...
Explanation:
We could write
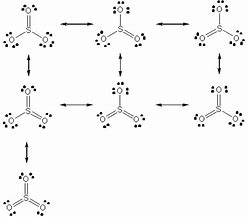
All of these structures are equivalent, and the similarly, the corresponding acid of

