In which of the following solution metallic ion can be displaced by aluminium : (i)CuSO4(ii)NaCL(CaCl2(IV)MgSO4 ?
1 Answer
Oct 5, 2016
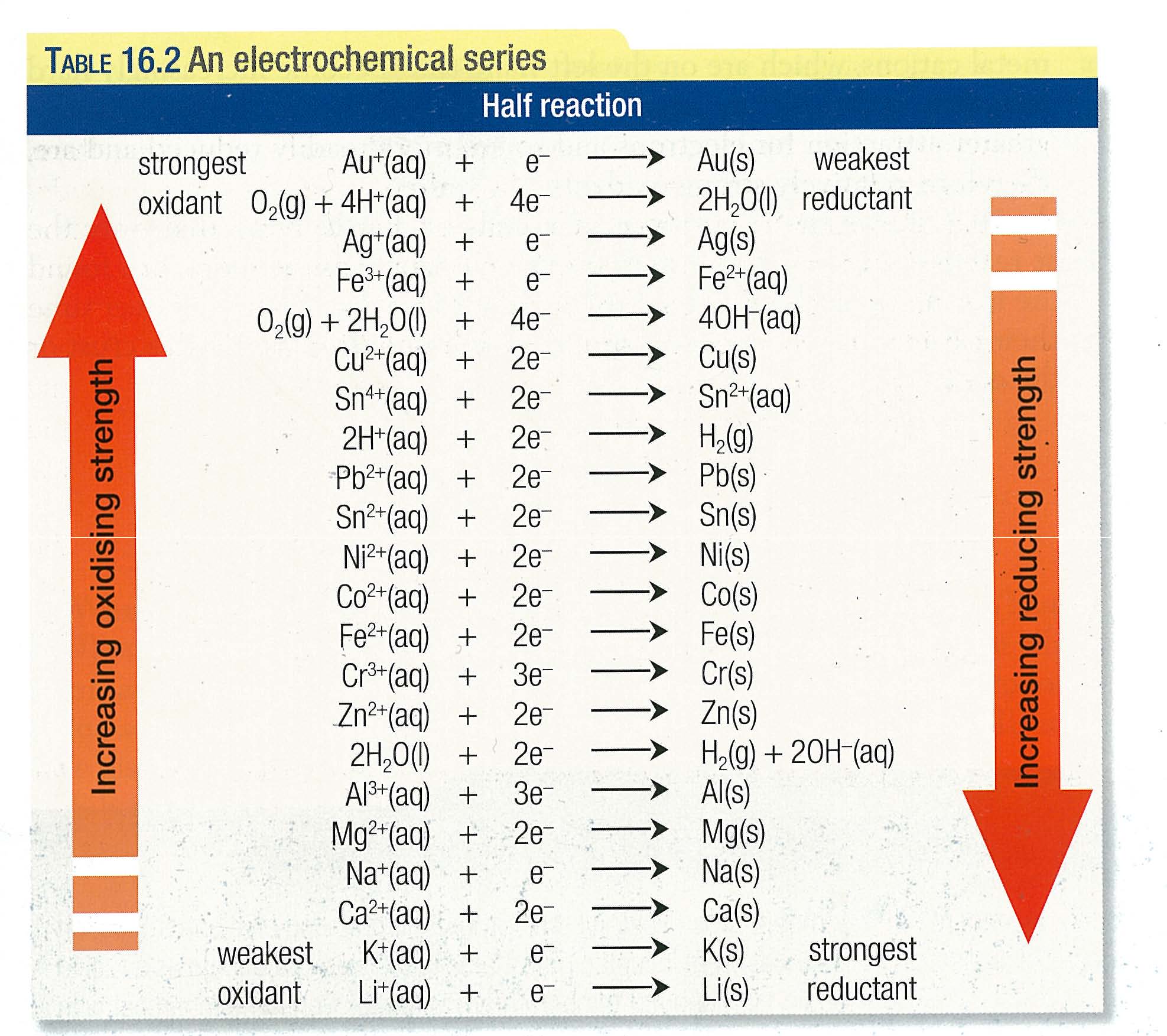
The ions present in solutions are
The metal dipped in solutions is
As per the electrochmical series the order of reducing strength of metal is
So Al can only reduce

