Is a solution of a weak acid considered a buffer solution?
1 Answer
Mar 24, 2018
No....
Explanation:
A buffer solution is composed of a weak acid, AND its conjugate base in
When a weak acid is titrated with a strong base, we inevitably get mixtures of weak acid with its conjugate base, and so this section of the titration curve is known as the
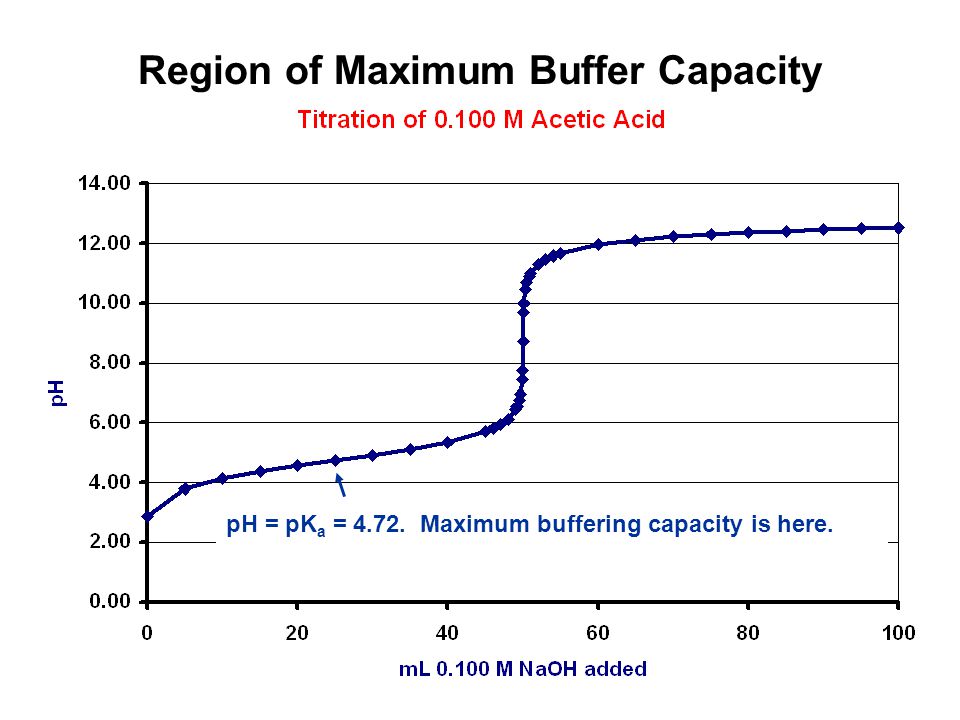
Biological systems are extensively buffered, including our own blood, and stomach acids....

