No, it couldn't be. It would have to donate an electron pair to be a Lewis base, or accept a proton to be a Bronsted base. It cannot do either.
A Lewis base must be able to donate an electron pair...
#"BH"_3# contains #3 + 3xx1 = 6# valence electrons: #2# per single bond.
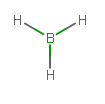
Therefore, #"BH"_3# is a trigonal planar molecule, which only has three electron groups. Of course, #"3 electron groups"# #-# #"3 bonds" = 0#, so it has no electron groups remaining that aren't bonded.
Furthermore, despite having a spare #2p_z# orbital, it's empty---it contains no electrons:
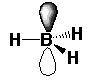
So, #"BH"_3# cannot be a Lewis base.
A Bronsted base must be able to accept a proton, i.e. an #"H"^(+)#, like this:
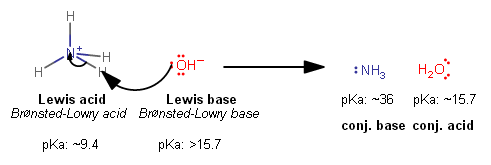
An #"H"^(+)# has no electrons to donate, so this is done by having the so-called Lewis base donate an electron pair, which is not possible for #"BH"_3# to do...
Hence, it is not a Bronsted base either.

