Is iron reactive?
1 Answer
Mar 25, 2018
Long answer short, not very - but compared to other common metals, yes.
Explanation:
Iron, chemically speaking, is quite low on the full reactivity series (see below), ranking only just above copper.
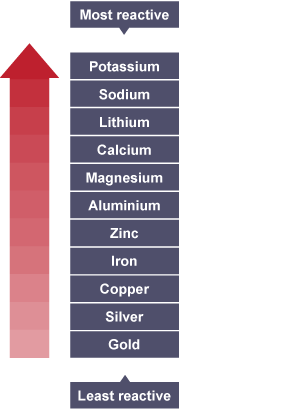
From the atomic structure of iron, its reactivity is mediocre, having two possible forms of bonding in its excited electronic state. However, due to the energy level of the electronic formation, it is very easily created (refer to any Astronomy textbook for star formation) and hence is the second most abundant metal in the Earth's crust and the fourth most abundant element on Earth.
Iron is also very easily oxidised by other elements (most of the time), hence iron is naturally found in the form of compounds when mined.