Is it true that the value of k depends on the amount of reactants and products mixed together initially?
1 Answer
Jun 6, 2018
No in either case...
- If you mean the rate constant
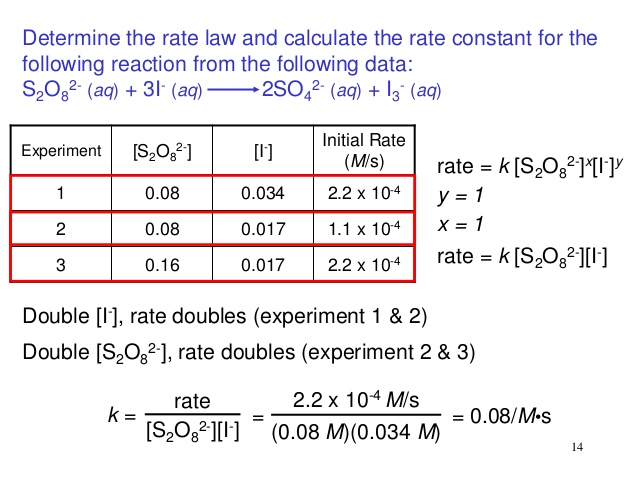
#k# , then no, it does NOT depend on concentrations at ANY time in the reaction. When we say it is constant, we mean it is constant with respect to concentration, i.e. it is NOT a function of concentration.
That is why you would get the same rate constant (more or less) if you calculate it using any (proper) trial, utilizing the reaction rate and concentrations found in that trial.
- If you mean the equilibrium constant
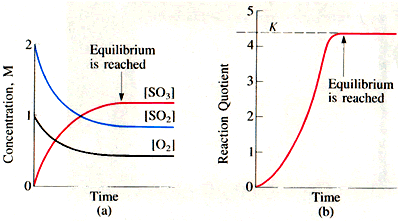
#K# , then still no, it does NOT depend on the initial concentrations. It only depends on the concentrations upon reaching equilibrium, i.e.
#lim_(t->oo) Q = K#
#K = (["product"]^("coefficient")cdots["product"]^("coefficient"))/(["reactant"]^"coefficient"cdots["reactant"]^"coefficient")#
#Q# , however, would depend on initial (current) concentrations, and is a function of time (here, we mean that initial is when we initially observe the reaction, not necessarily at time zero).And
- if
#Q > K# , the reaction is product-favored and shifts towards the reactants to restore equilibrium.- if
#Q < K# , the reaction is reactant-favored and shifts towards the products to restore equilibrium.- otherwise (
#Q = K# ), the reaction is at equilibrium.

