Well, it's a gas... how do I know? I've smelled it passing by, after seeing a student react a mixture of cations (containing #"NH"_4^(+)#, #"Mn"^(2+)#, #"Fe"^(3+)#, #"Ag"^(+)#, #"Ni"^(2+)#, and #"Al"^(3+)#) with #"NaOH"#.
The #"NaOH"# reacted with the #"NH"_4^(+)# (and with some of the other cations) to produce a gas that smelled like cleaning products...
#"NH"_4^(+)(aq) + "NaOH"(aq) -> "NH"_3(g) + "Na"^(+)(aq) + "H"_2"O"(l)#
It formed as a gas, since its boiling point at #"1 atm"# is only #-33^@ "C"#. It spontaneously vaporizes under these conditions, if it were somehow obtained as a liquid.
A bit hard to read, but this is the best phase diagram I could find:
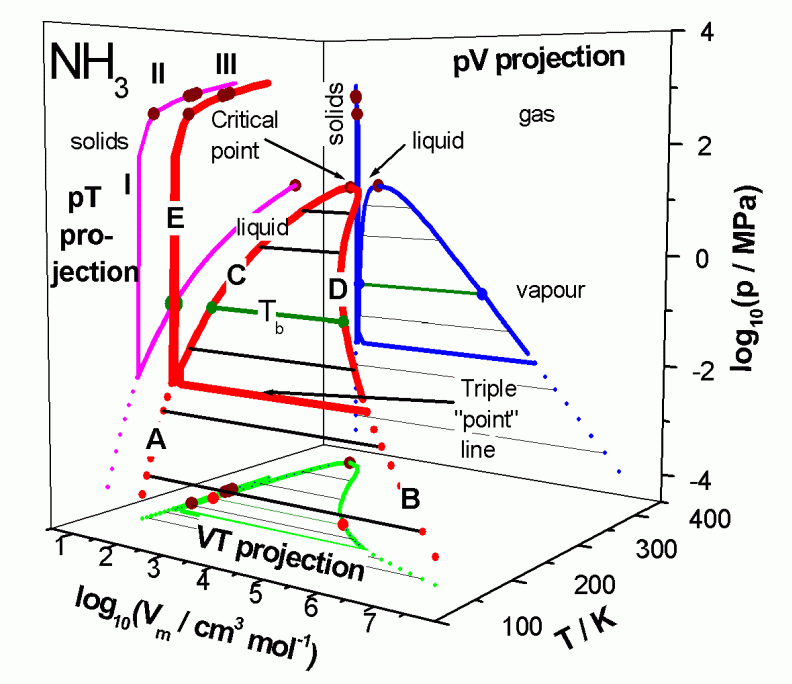
The #PT# projection (purple) shows that at #"1 atm"# (#log_10(P//"MPa") ~~ -1#) and #"300 K"#, we are beneath the bottom purple curve, which divides the liquid (above) from the gas (below) regions.
Hence, at #"1 atm"# and #"300 K"#, ammonia is certainly a gas.


