Is pressure of the gas on the wall of a vessel bigger if the gas is a real gas or an ideal gas? Explain?
1 Answer
Feb 11, 2017
To a first approximation, the pressure should be the same.......
Explanation:
Of course, real gases are not ideal gases, and in fact there are no such beasts as ideal gases. Real gases, whatever their identity, have some degree of interaction between particles, and depending on the circumstances, the experimental pressure should be slightly LESS than that predicted.
 coecs.ou.edu
coecs.ou.edu
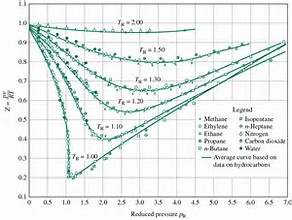
Sometimes the so-called compressibility of different gases are plotted, as in the diagram. Here the product of the

