Is the change in enthalpy positive or negative for an exothermic reaction?
1 Answer
Dec 5, 2015
Negative.
Explanation:
The change in enthalpy in an exothermic reaction is negative, since overall heat is lost ( "exo"thermic means that heat is leaving).
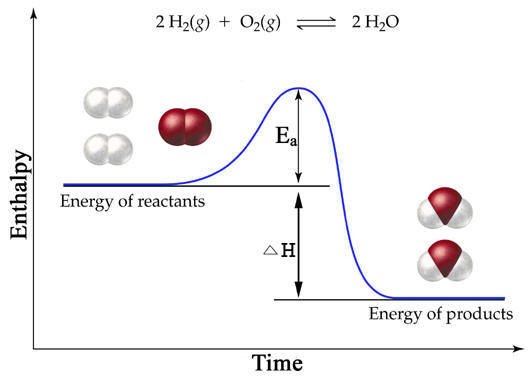
Notice how the total enthalpy decreases in this exothermic reaction.
The opposite of this would be a positive change in enthalpy during an endothermic reaction.

