Is the chlorination of methane a free radical halogenation?
1 Answer
Jun 10, 2015
Yes, the chlorination of methane is a free radical reaction.
Explanation:
There are three major steps in the reaction.
Step 1. Initiation
The initiation step involves the homolytic cleavage of a
chemistry2.csudh.edu
Step 2. Propagation
- A
"C" atom removes an"H" from methane, producing"HCl" and a methyl radical.
 upload.wikimedia.org
upload.wikimedia.org
- The newly-formed methyl radical abstracts a
"Cl" from a chlorine molecule, producing chloromethane and re-forming a"Cl" atom.
Step 3. Termination
In the termination steps, the radicals combine in all possible combinations.
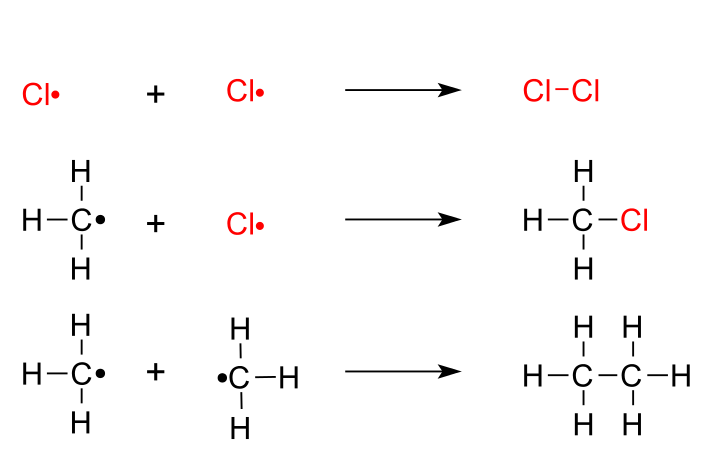 upload.wikimedia.org
upload.wikimedia.org
The termination products are chlorine, chloromethane, and ethane.

