Is there any sort of resonance in this ion?

1 Answer
Yes,there is resonance in this ion.
But it isn't the type you may be thinking of.
It is just the normal resonance involving the two Kekulé forms of benzene.
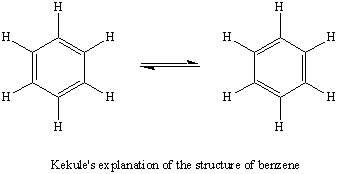 www.kentchemistry.com
www.kentchemistry.com
It doesn't involve the lone pair of electrons on the carbon atom.
 C6H5-
C6H5-
If you think about it, you will realize that the anion was formed by removal of a proton like the one indicated in the picture below.
 sp2
sp2
(Adapted from jayzeetee.wordpress.com)
The lone pair is in an
That means there in no interaction between them
If the major lobe of the
There is no net interaction and therefore no resonance involvement of the

