Is there a formula for kinetic theory of gases (the collision with a wall)? If there is, how can I derive it?
How can I show the basic steps how that formula was made?
How can I show the basic steps how that formula was made?
1 Answer
Consider a hard-sphere model for an ideal gas (call it molecule 1) traveling at velocity
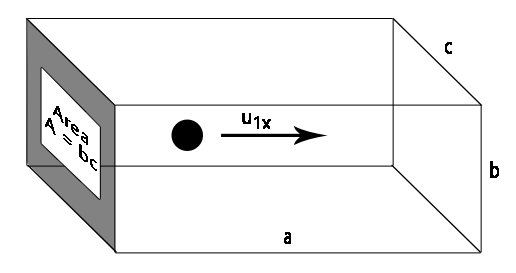
The momentum of the particle is then
Exactly when the particle collides with the wall, we assume the collision is elastic (no kinetic energy is lost), and that the new momentum after hitting the wall becomes
Therefore, the change in momentum over time, starting at the moment the particle hits the rear wall and bounces back, hits the front wall, and returns to the rear wall without colliding again, becomes:
#(Deltap_x)/(Deltat) = (Delta(m u_(1x)))/(Deltat) = (m u_(1x) - (-m u_(1x)))/(Deltat)#
The velocity was
#u_(1x) = (2a)/(Deltat)# ,
so that
#=> (2m u_(1x))/(2a"/"u_(1x)) = (m u_(1x)^2)/a#
Recall that
#(Deltap_x)/(Deltat) = F_1 = (m u_(1x)^2)/a#
Also recall that the pressure exerted on the rear wall is
#P_1 = F_1/(bc) = (m u_(1x)^2)/(abc) = (m u_(1x)^2)/V# ,where
#V = abc# is the volume of the container.
When we expand this expression to consider all molecules colliding with the same wall, we obtain:
#P = sum_(j=1)^(N) P_j = sum_(j = 1)^(N) (m u_(jx)^2)/V = m/V sum_(j=1)^(N) u_(jx)^2# where
#N# is the total number of molecules.
Note that
#P = m/V sum_(j=1)^(N) u_(jx)^2 = m/V N * 1/N sum_(j=1)^(N) u_(jx)^2#
#=> PV = Nm<< u_x^2 >>#
Although we chose the x direction in the derivation, we could have chosen any arbitrary direction (a homogeneous gas is "isotropic"; it has the same properties in any direction).
So,
#u^2 = u_x^2 + u_y^2 + u_z^2# ,
so the average value of the total speed squared is then:
#<< u^2 >> = << u_x^2 >> + << u_y^2 >> + << u_z^2 >>#
Therefore, since the averages of the squared speed in each dimension are all equal, if we average the three average squared speeds in each dimension, we get the average of the squared speed in any arbitrary direction,
#=> color(blue)(PV = 1/3 N m << u^2 >>)#
Lastly, note that

