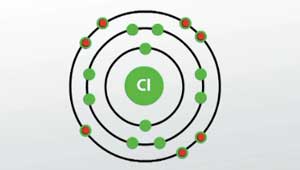
Is this bohr atomic structure of Cl right??
2 Answers
No I don't believe so.
Explanation:
The two electrons in the first shell should be together not single. and the third shell with 7 electrons should have three sets of doubled electrons and only one single electron.
I believe so.
Explanation:
This is a Bohr model of a chlorine-35 atom. Some Bohr models pair six of the seven electrons in the third (valence) shell. This helps to see that one valence electron is available for bonding. From my research, Bohr models of Cl-35 either have the electrons in the second and third shells paired, or they don't have any paired electrons in the first, second, and third shells. The model does show that there are two electrons in the first shell, eight electrons in the second shell, and seven electrons in the third shell, which is correct.
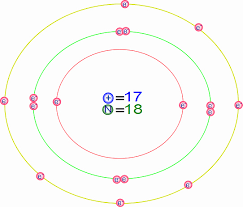
Below are several examples of Bohr models of chlorine atoms.