Is this phrase correct? "average kinetic energy isn't so hot"
1 Answer
No. It should be, "average kinetic energy isn't so [high]". Since
From this video, the context is that:
- The temperature is proportional to the average kinetic energy, i.e.
#K_(avg) prop T# . - The average kinetic energy of an ensemble of molecules allows individual molecules to have varying kinetic energies amongst themselves, and this variance can be large or small.
Since we're talking about a distribution of kinetic energies when we say "average kinetic energy", the speaker was saying that some molecules at the surface of a solution will have enough kinetic energy to escape into the air as gas molecules, breaking free of the intermolecular forces that held the liquid together.
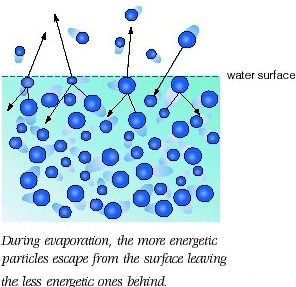
In short, he is saying that even at room temperature (more specifically, not at the normal boiling point), when "the average kinetic energy isn't so [high]", evaporation occurs without a problem.
In other words, he was explaining why evaporation occurs at room temperature, and we don't have to boil a solution in order for vaporization to occur.

