Is warm air high or low pressure?
1 Answer
High.
Explanation:
People sort of get confused by this one because they always think that warm air rises. There are several gas laws you need to understand however.
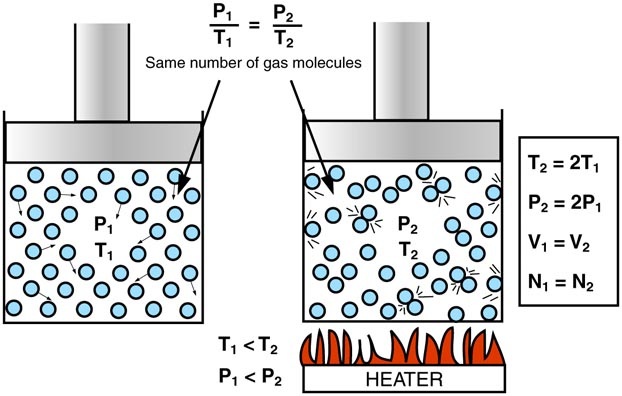
The first is Gay-Lussac's law which states that pressure and temperature are proportional for constant volume. Think about how a pressure cooker works or heating a tank of compressed air and this should totally make sense.

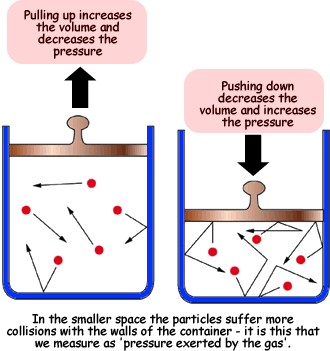
The next is Boyle's Law which states that pressure and volume are indirectly proportional for a constant temperature. This makes sense if you think about a balloon. The only way that you can decrease the volume of a balloon at a constant temperature is if you squeeze the balloon, which is increasing the air pressure.

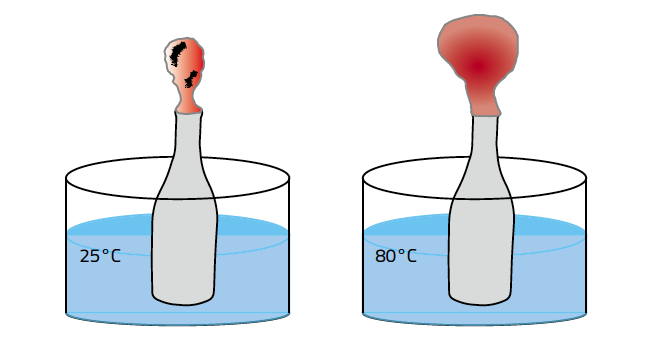
The last law to consider is Charles' Law which states for a constant pressure, volume and temperature are directly proportional. In other words when you apply heat to a balloon, in order for the pressure to remain constant the balloon has to expand.

So what you have to ask yourself in any given situation, is the air free to expand. In the case of air at the equator, all the air is being heated and therefore all the air is trying to expand. Since it is all trying to expand it can't expand and so the pressure increases. The pressure is always higher at the equator than at the poles. At any give local, if one spot is heated more than the areas around it that air can expand. In this case the volume of the air increases with temperature and therefore the pressure decreases.
Another way to look at it is; warming air does not rise, air that is increasing in volume is what rises.

