MECHANISM of electrophilic substitution reaction OF 4-Methylphenyl benzoate ?
1 Answer
Jun 25, 2018
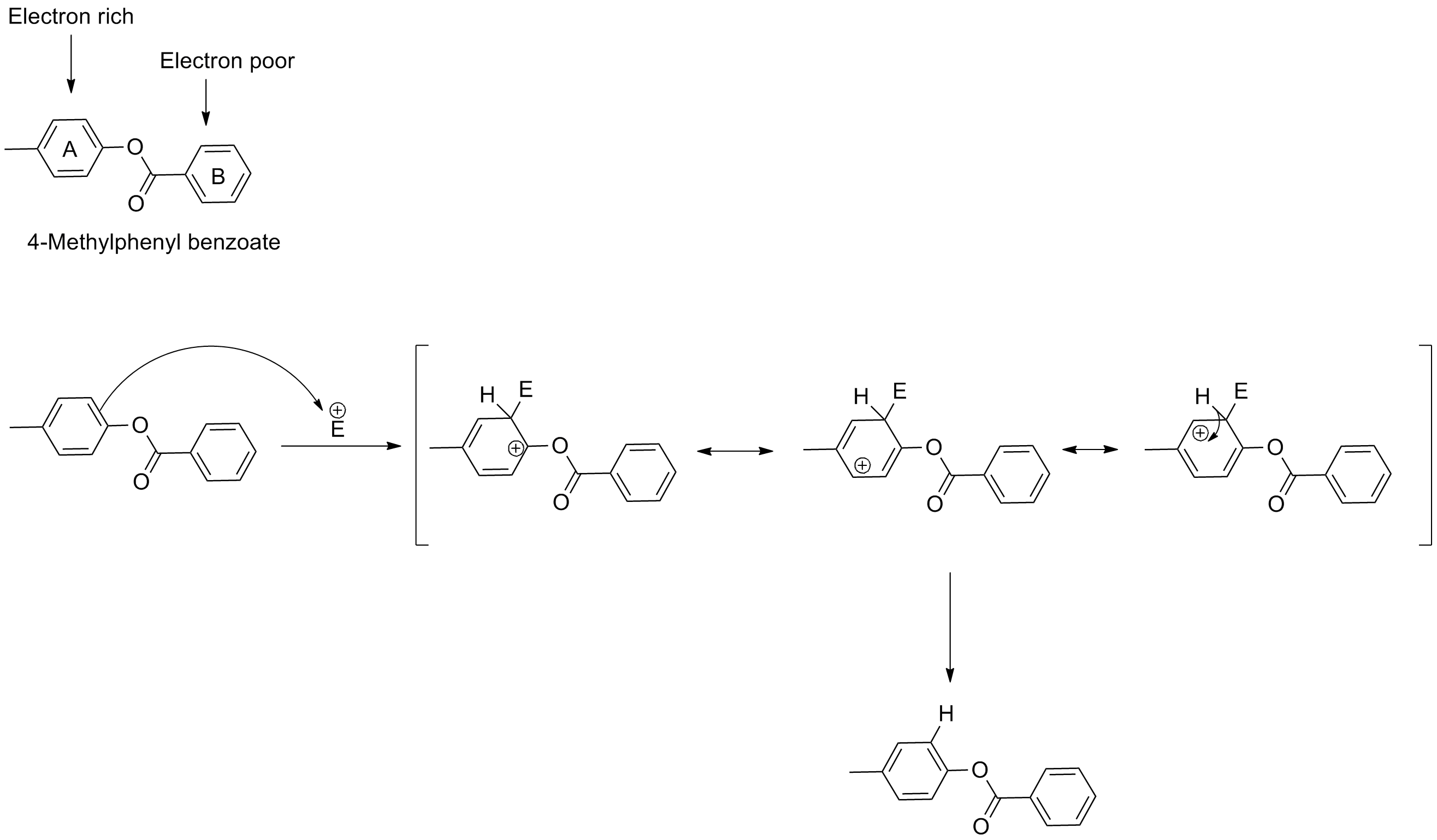
Explanation:
Electrophilic substitution occurs in the ring with higher electron density. In the given compounds ring A is electron rich due to the presence of electron donation groups (methyl and benzoloxy). So, reaction occurs at ring A. Benzoyloxy group is more electron donating than methyl, so, substitution occurs ortho to the benxzoyloxy group.

