Mg was reacted with an excess of HCl dilute and the #H_2# gas produced collected in an eudiometer. The volume of hydrogen in the eudiometer was corrected to conditions of STP. If 94.1 milliliters of H2 was produced, how much Mg reacted in this experiment?
1 Answer
Given
Explanation:
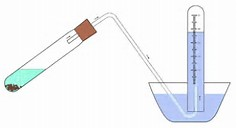
A
The stoichiometric equation EXPLICITLY shows stoichiometric equivalence between the metal and dihydrogen.
(And if you think all this bumf in converting to LESS than 1 standard atmosphere is a bit tedious, I agree with you!).
Given the stoichiometry, we use approx.

