(Na,P,Cl,Si,Mg )which of these has largest atoms?
1 Answer
Here is an important Periodic Trend that you should commit to memory....
Explanation:
Why? The size of an atom is reasonably the radius of its valence electron(s). This radius is (i) a function of
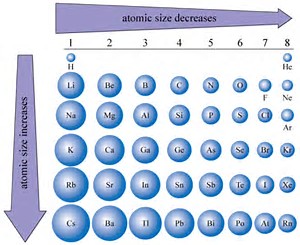 Wikipedia.org
Wikipedia.org
Does this Table support what I have argued? Why or why not?
And so your biggest ATOM is CLEARLY sodium...you can order the sizes of the rest of the atoms...

