Add this so-called #"aliquot"# to a #100*mL# volumetric flask....and make up the volume to #100*mL# with distilled water...
And the new concentration is, always, #"moles of solute"/"volume of solution"#
#=(10*mLxx10^-3*L*mL^-1xx0.40*mol*L^-1)/(100*mLxx10^-3*L*mL^-1)#
#0.040*mol*L^-1#...the which is ONE TENTH of the original concentration as required.
And when you use volumetric glassware, you recall that the meniscus of the solution is conceived to just touch the required graduation...
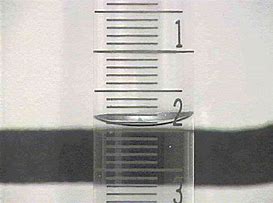
...I would call the given reading at #1.91*mL#... I could not find a decent illustration of a filled pipette.. But remember these are VERY accurate pieces of kit, and you can get very impressive, reproducible results with same and a bit of attention to detail and technique....


