O2 molecules there are 1 sigma bond and 1 pi bond and 2 lone pair of electron for each oxygen ( non bonding electrons ) ?? so how MOT diagram shows 1 sigma and 2 pi bond and there is no non bonding electrons as all orbital combine together ?
1 Answer
It should all check out. You just have to reinterpret the MO diagram of
The MO diagram of
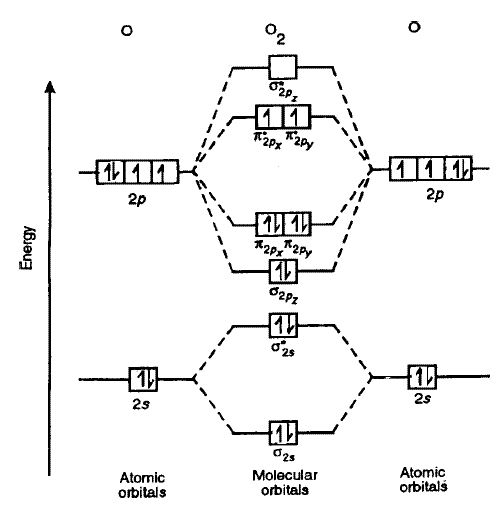
for the Lewis structure:
#:ddot"O"=ddot"O":#
The nonbonding electrons come from filled bonding AND antibonding MOs at the same time. So, what you should be looking at to correlate these is:
#sigma_(2s)# and#sigma_(2s)^"*"# (both electrons in each orbital)#pi_(2p_x)# ,#pi_(2p_y)# ,#pi_(2p_x)^"*"# , and#pi_(2p_y)^"*"# (only one electron in each orbital)
There isn't always a neat correlation like this, but this gives
#ul(color(blue)bb(uarr) color(white)(darr))" "ul(color(blue)bb(uarr) color(white)(darr))#
#pi_(2p_x)^"*"" "" "pi_(2p_y)^"*"#
#ul(color(blue)bb(uarr) darr)" "ul(color(blue)bb(uarr) darr)#
#pi_(2p_x)" "" "pi_(2p_y)#
#ul(uarr darr)#
#sigma_(2p_z)#
#ul(bbcolor(blue)(uarr darr))#
#sigma_(2s)^"*"#
#ul(bbcolor(blue)(uarr darr))#
#sigma_(2s)#
That leaves the two

