Organic Chemistry Help Please? D:
2 Answers
Explanation:
This is an oxidation reaction. Adding oxygen means oxidation, adding hydrogen would be a reduction reaction. If you look at the structure of toluene below on the left and benzoic acid on the right, you can see that we need to add in two oxygen atoms.
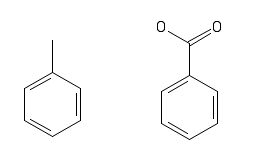 https://pubchem.ncbi.nlm.nih.gov/edit2/index.html
https://pubchem.ncbi.nlm.nih.gov/edit2/index.html
So you need an oxidant, something with a source of oxygen. The
In this case a strong oxidising agent such as acidified
An interesting thing to note is that toluene is not miscible in water. But it is slightly soluble and possibly more soluble in acidic solution.
We could write separate redox reactions....
Explanation:
Dichromate is reduced to
And toluene is oxidized to benzoic acid...
And we add the equation together to eliminate the electrons...
So we use the acidified sodium dichromate...and we observe a change in colour from the orange-red of dichromate to the green chromic ion...


