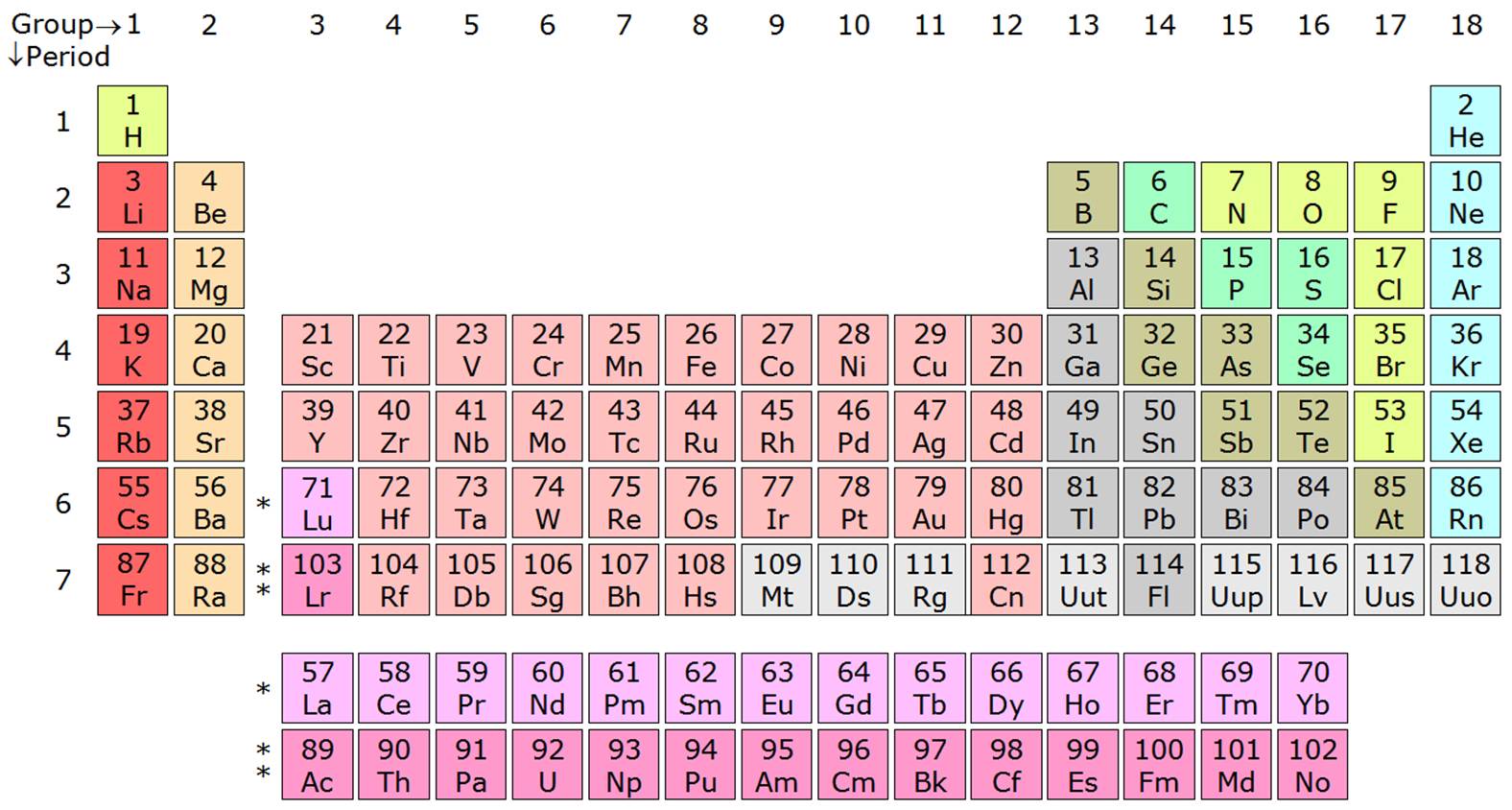
Out of #Hf, Cs, Pb, Pt #, which element has the smallest atomic radius?
1 Answer
Nov 10, 2016
Explanation:
Hf, Cs, Pt and Pb all lie in the same period, i. e. sixth period.
Across a period (left to right) the atomic size decreases .... due to increase in effective nuclear charge.
soooo.... Pb has the smallest atomic radius and Cs is the largest among the four given

