Phosphorus-32 has a half-life of 14.0 days. Starting with 6.00 g of #""^32P#, how many grams will remain after 56.0 days?
Express the answer in numerically in grams.
Express the answer in numerically in grams.
2 Answers
May 17, 2018
Explanation:
And
And given that the initial quantity was
May 17, 2018
The mass remaining is
Explanation:
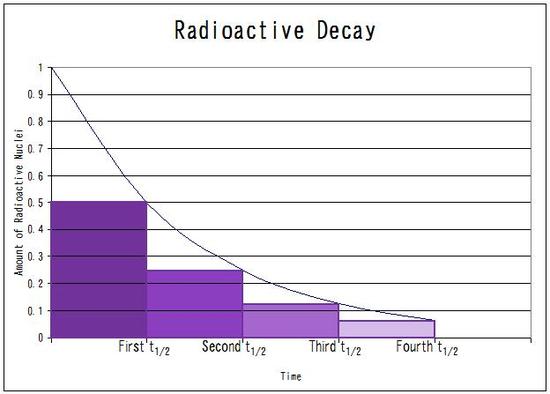
The half life is
The number of half life of
The initial mass of
After each half life, the mass will decrease by
Therefore,
the mass remaining is


