Please explain me iiiB group of the periodic table.?? Thank you.
1 Answer
Jul 19, 2017
It is usually considered to contain the entire
But, it seems that group
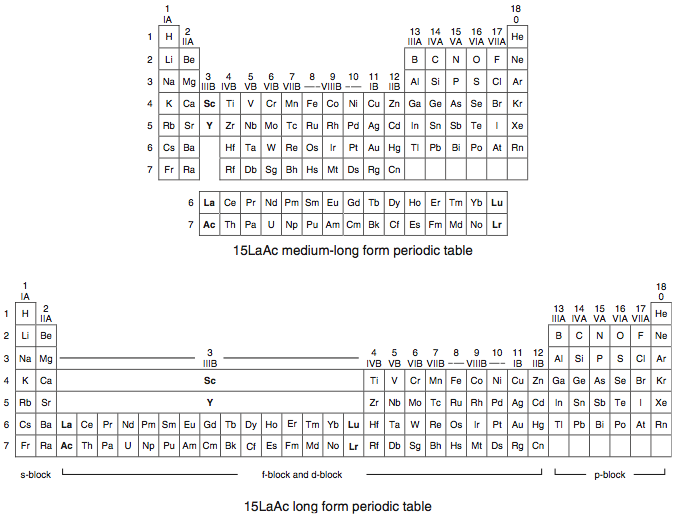
Another periodic table shows:
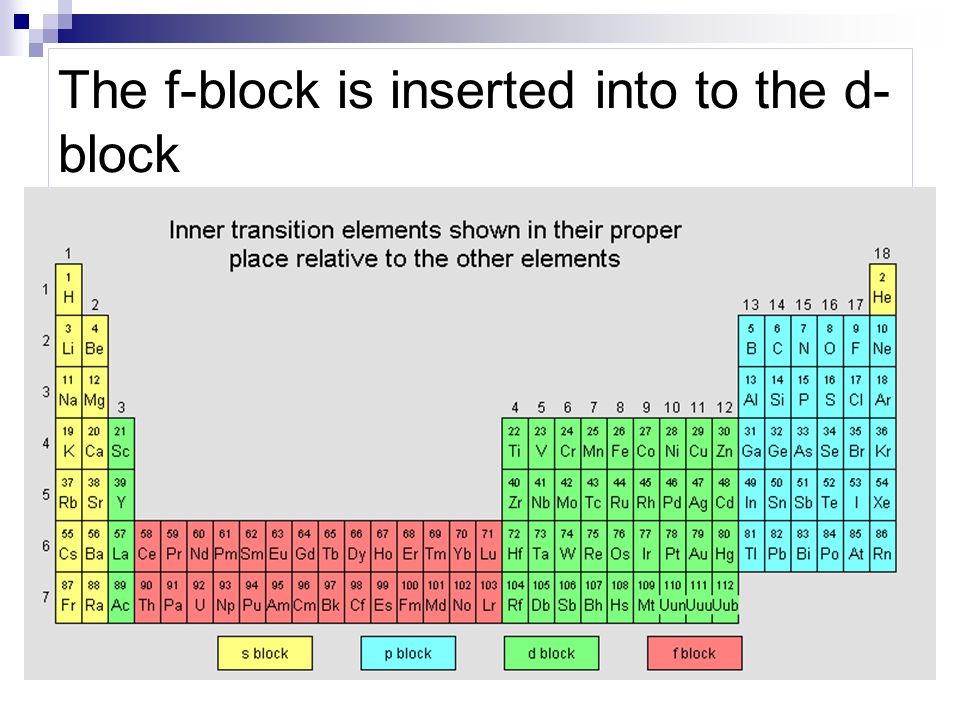
and another one shows:
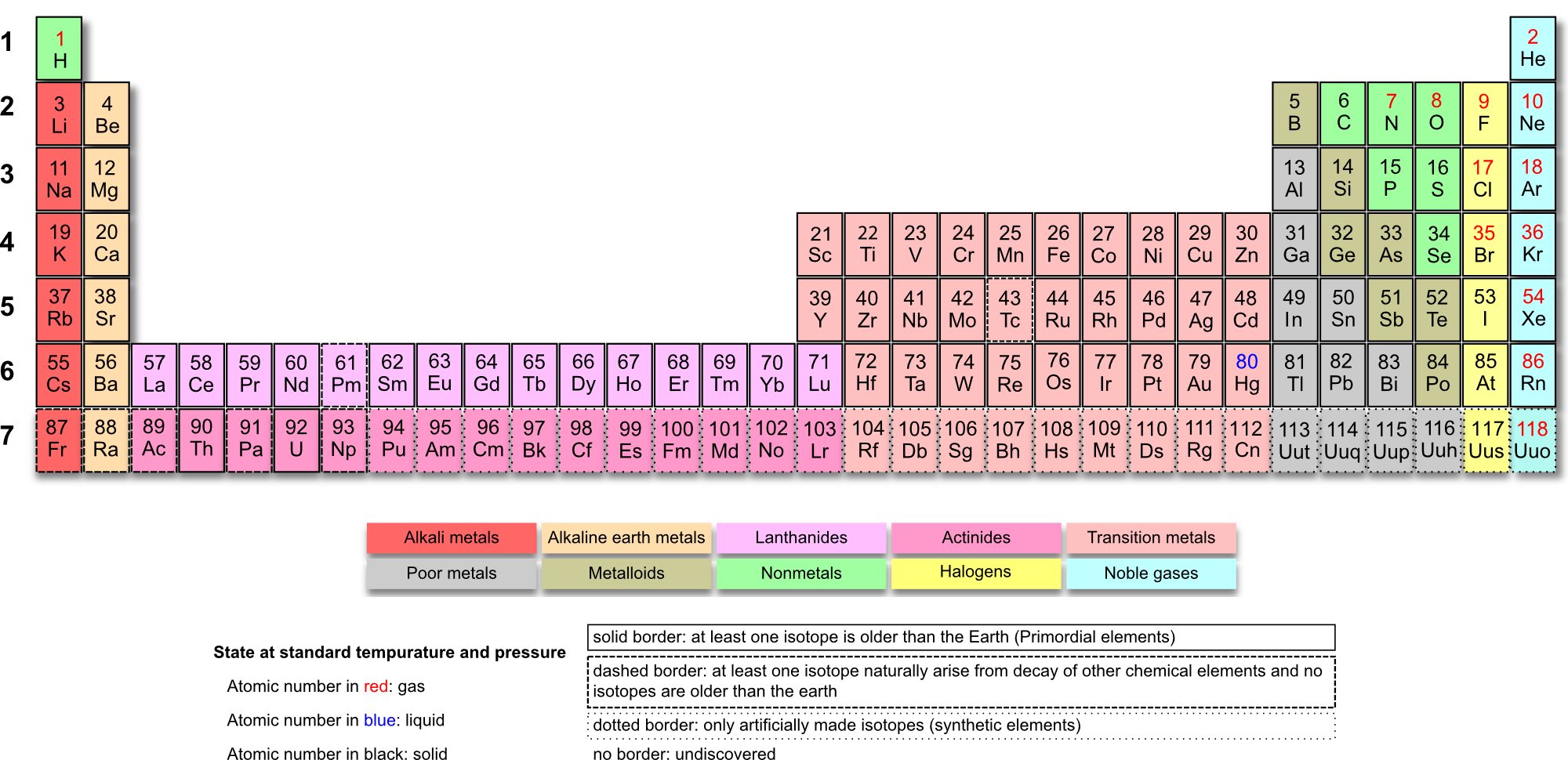
either of which would have shown the first or last

