Please explain the nomenclature of coordination compounds??
1 Answer
For relatively simple compounds, it is basically alphabetical ordering of ligands, with prefixes just like in general chemistry.
The main differences are that things connected to the central atom can be more complicated than simple atoms, and that there are new prefixes for complicated ligands to make names look nicer.
DISCLAIMER: LONG REFERENCE ANSWER!
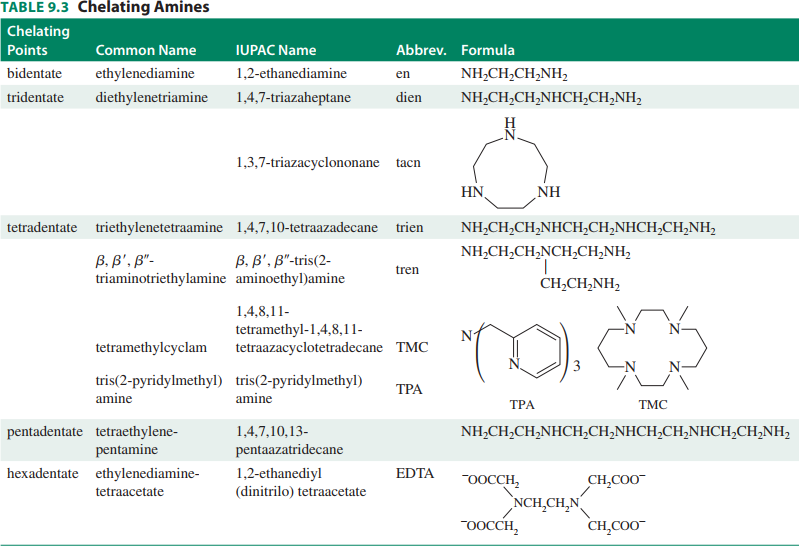
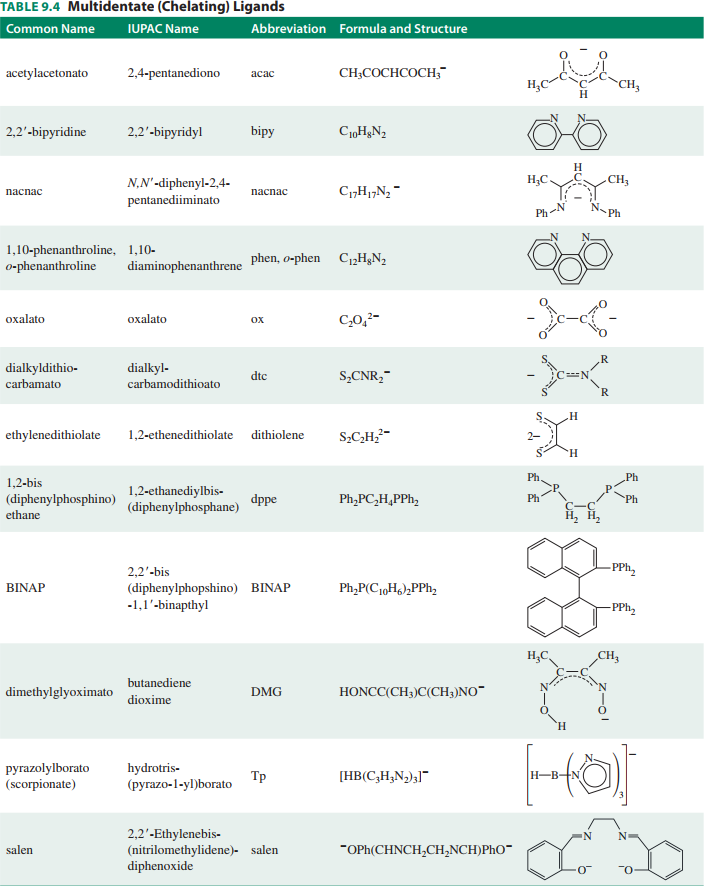
See the bottom of the answer (Appendix I, II) for prefixes and lists of ligands to know or be aware of.
SIMPLIFIED NOMENCLATURE RULES
- List ligands alphabetically.
- List the metal name last, along with its oxidation state.
- As usual, put the cation name first and put a space after it.
- If the metal is within an anionic complex, then use the name of the metal that matches the periodic table symbol and end with "-ate".
- Use prefixes from table
#"I"# (Appendix I) to indicate multiple identical simple ligands. - Use prefixes from table
#"II"# (Appendix I) to indicate multiple identical complicated ligands.
ISOMERIZATION RULES
There are also isomers: cis/trans (octahedral and square planar),
- cis/trans indicates ligands on the same/opposite side of the metal center.
#Delta//Lambda# indicates that for octahedral complexes with chelating ligands, the axial-equatorial "fin" is facing the right/left when the equatorial "fin" faces the back.- fac/mer indicates that three identical ligands form an octahedral "face", or form a "meridian" respectively.
There are other isomer rules, but we will not list them here.
BINDING RULES
- If there is a bridging ligand, indicate it using
#mu_x# (mu-x), where#x# is the number of metal centers the ligand is bridging across. - If there is a non-innocent ligand (such as nitro, or thiocyanato/isothiocyanato), indicate it using
#kappa-X# (kappa-X), where#X# is the atom that the ligand binds through. - If there is a ligand that binds via multiple atoms (a non-unit hapticity), indicate it using
#eta^n# (eta-n), where#n# is the number of atoms that bind to the same metal center.
SIMPLE (UNDERGRADUATE) EXAMPLES
#["Ag"("NH"_3)_2]^(+)#
- diamminesilver(I)
Note that ammine has two#m# 's, but ethylenediamine has one#m# . The "di" indicates two ammine ligands.
#["Ti"("CO")_6]^(2-)#
- hexacarbonyltitanate(II)
Note that the ending is titanate, not titanium, because the complex has a#2^-# charge. "Hexa" indicates six identical ligands.
#"K"_4["Fe"("CN")_6]#
- potassium hexacyanoferrate(II) [not ironate]
Note the similarity to general chemistry ionic compound naming. The cation name is placed first, with a space. Also, the name of iron as seen in the periodic table symbol is used (#"Fe"^(2+//3+) harr "ferro/i"# ), not ironate.
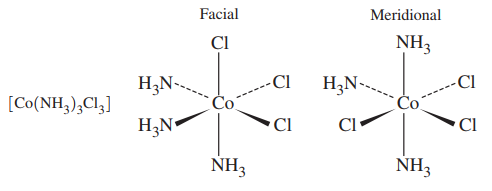
- fac/mer-triamminetrichlorocobalt(III)
The left isomer forms octahedral faces, and the right isomer forms meridional semicircles on the#xy# and#yz# planes (right-handed coordinate system).
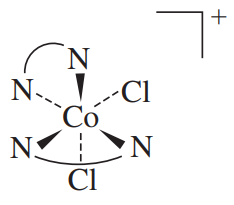
#Delta# -cis-dichlorobis(ethylenediamine)cobalt(III)
Note that here,#Delta# is needed to indicate that if the equatorial#"en"# fin was in the back, the axial-equatorial#"en"# fin would face to the right.
Also, the#"Cl"^(-)# are on the same side to each other, so we label that as "cis-dichloro".
Lastly, bis was the prefix needed since "di" was already in the ligand name of#"en"# .
ADVANCED (UNDERGRADUATE/GRADUATE) EXAMPLES
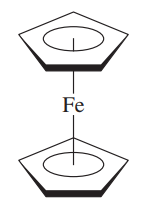
ferrocene OR
bis(#eta^5# -cyclopentadienyl)iron(II)
The first is a common name for the metallocene class of compounds. The second is the more formal name, indicating that there are two#"C"_5"H"_5# rings bound to iron via all 5 of their carbon atoms each.
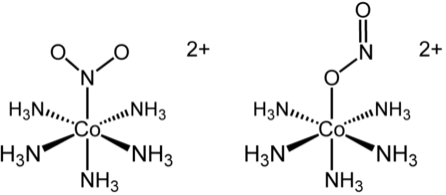
pentaammine(nitrito-
#kappa"-N"# )cobalt(III)
if#"NO"_2^(-)# binds via nitrogen atom
pentaammine(nitrito-#kappa"-O"# )cobalt(III)
if#"NO"_2^(-)# binds via oxygen atomIf we simply said "nitro" instead of "nitrito-
#kappa# -#X# ", it would not be clear which atom the#"NO"_2^(-)# binds through.
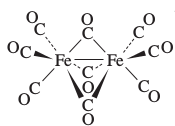
diiron nonacarbonyl OR
tri-#mu_2# -carbonyl-bis(tricarbonyliron)(Fe(0)-Fe(0))The first name is informal, only telling you how many of each thing there are.
The second name tells you everything you need to know: there are three#"CO"# ligands (tri) that bridge across two metal centers (#mu_2# ), connected on either side to two#"Fe"("CO")_3# fragments (bis(tricarbonyliron)). The iron centers are usually connected, and (Fe(0)-Fe(0)) emphasizes that.
APPENDIX I --- PREFIXES
TABLE I
#ul("Prefix"" "" ""Number of Identical Ligands")#
#"N/A"" "" "" "1#
#"di-"" "" "" "color(white)(i.)2#
#"tri-"" "" "" "color(white)(ii)3#
#"tetra-"" "color(white)(i.....)4#
#"penta-"" "color(white)(i....)5#
#"hexa-"" "color(white)(i.....)6#
#ul(vdots" "" "" "color(white)(i.)vdots" "" "" "" "" "" "" "" "" "" ")#
TABLE II
#ul("Prefix"" "" ""Number of Identical Ligands")#
#"N/A"" "" "" "1#
#"bis-"" "" "" "color(white)(.)2#
#"tris-"" "" "" "color(white)(i)3#
#"tetrakis-"" "color(white)(ii)4#
#"pentakis-"" "color(white)(i)5#
#"hexakis-"" "color(white)(i.)6#
#vdots" "" "color(white)(iii..)vdots#
APPENDIX II --- LIGAND LIST
Here is a list of common monodentate ligands to memorize!
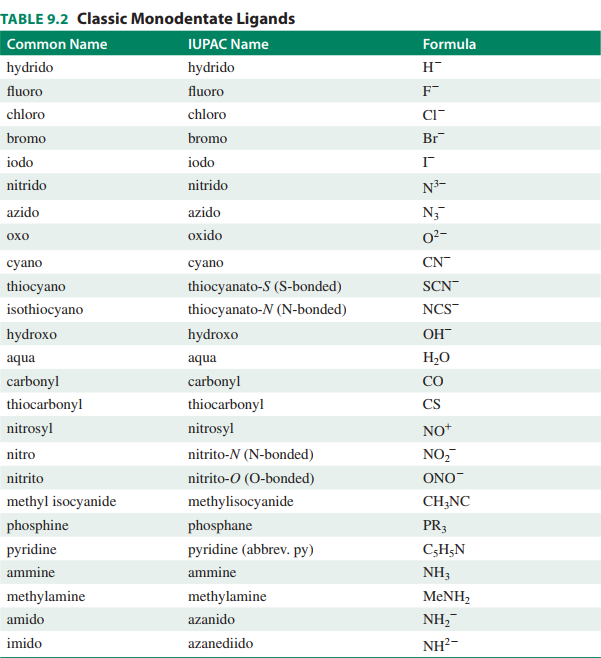
Here is also a list of polydentate ligands to be aware of (but not memorize):