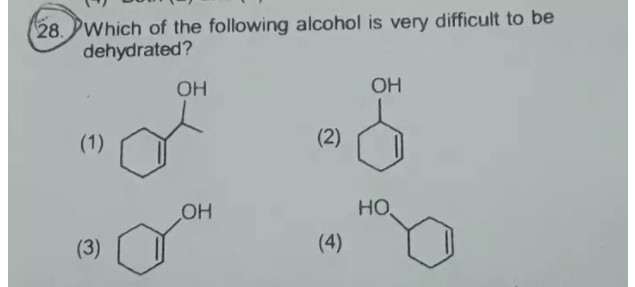
Please give the proper reason with details in this question?
1 Answer
Well, in a dehydration reaction, remember that the
So the one that is hardest to dehydrate is the one that
-
(1) could work just fine to generate api bond adjacent to the"OH" ; there are threebeta hydrogens to its right. -
(2) is possible, but the twobeta hydrogens to the left of the"OH" is preferentially chosen since forming"C"="C"="C" (by choosing the right-handbeta hydrogen) is not as easy as forming conjugatedpi bonds. -
(4) is arguably the easiest, because it has four adjacentbeta hydrogens, two on each side of the"OH" .

