Please let me know why it is necessary to scrupulously oven-or flame-dry the glass used in Grignard reaction? Please write the reaction.
1 Answer
Sep 6, 2015
Grignard reagents are highly sensitive to water. If you add water, then the magnesium acquires a hydroxide while the original grignard reagent acquires a proton instead, and gets deactivated.
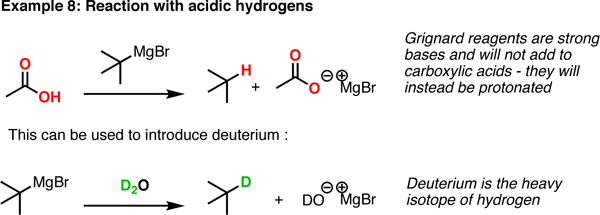 http://masterorganicchemistrycom.c.presscdn.com/
http://masterorganicchemistrycom.c.presscdn.com/
Look at the bottom reaction here, except replace
Note: This tends to be part of a lab you do early in the year for Organic Chemistry II. I think you should ask your professor if you have questions about your lab, because they will be around.

