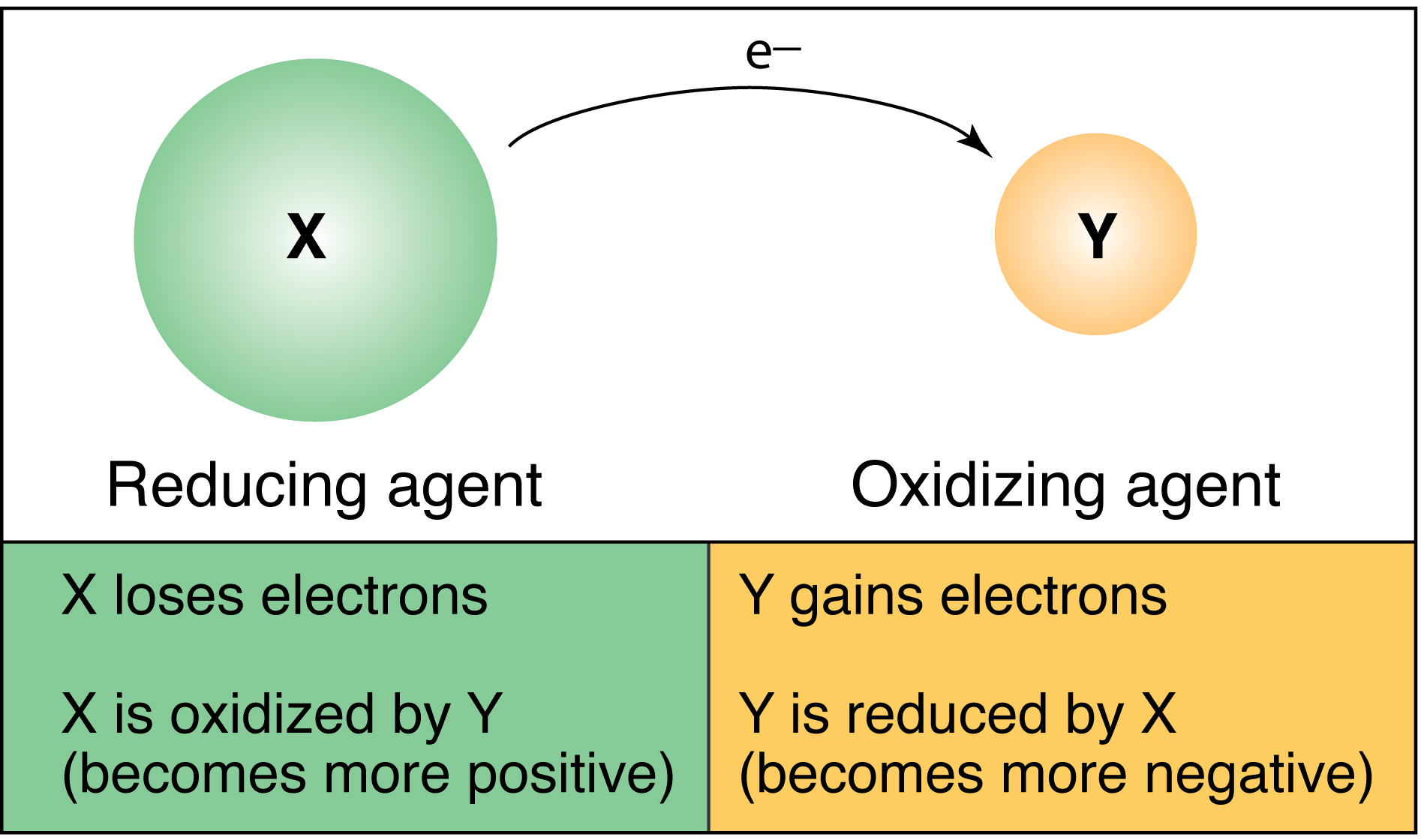
In the reaction 2"FeCl"_2 + "Cl"_2 -> 2"FeCl"_32FeCl2+Cl2→2FeCl3, chlorine may be regarded as?
a)a) an oxidizing agent
b)b) a reducing agent
c)c) a catalyst
d)d) providing an inert medium
1 Answer
Jun 27, 2018
The answer is
Explanation:
 sites.goggle.com
sites.goggle.com
The reaction is
The reactions are
So, Chlorine is the oxidising agent.
The answer is

