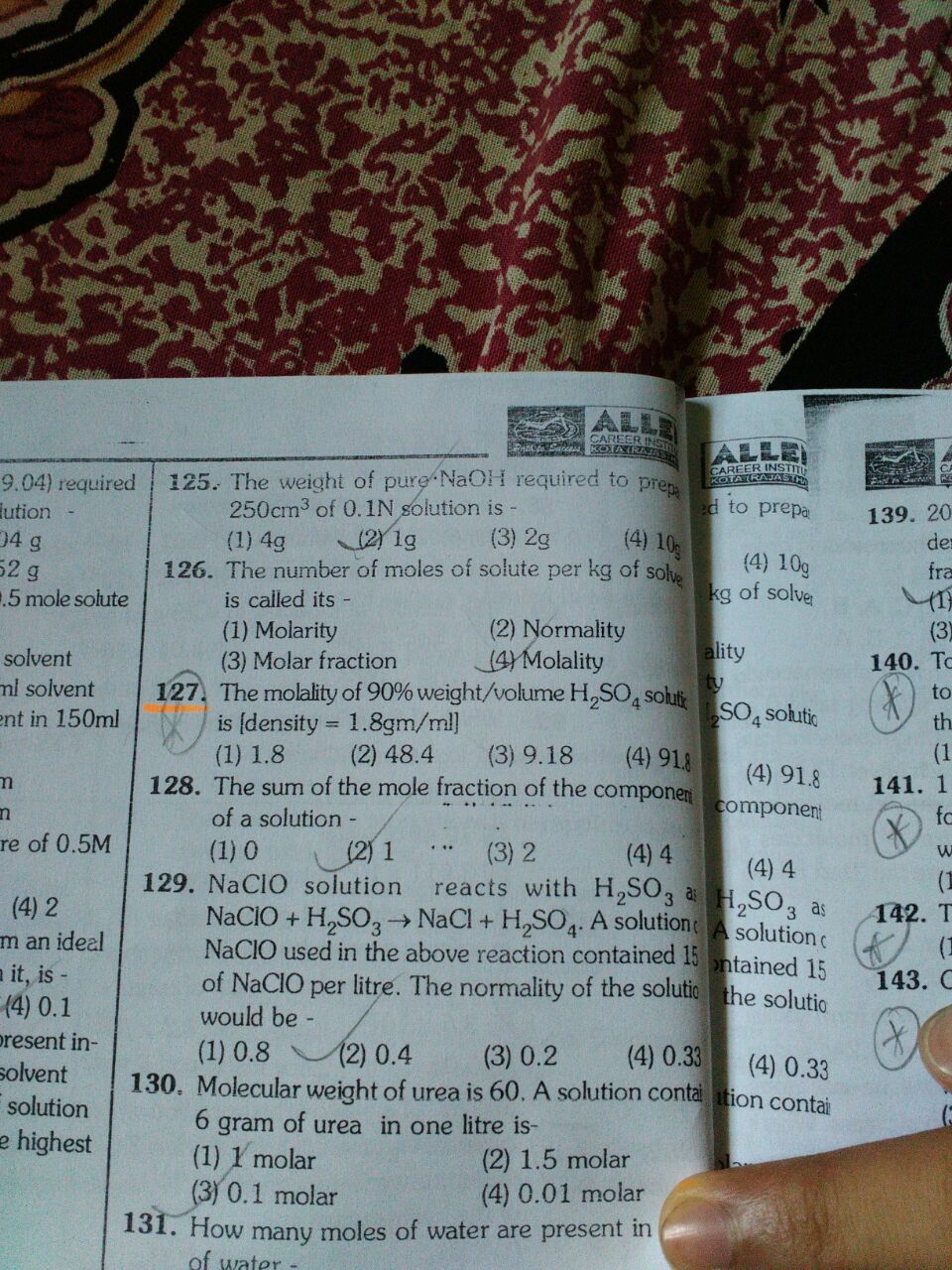
Please solve q 127?
2 Answers
Please see the explanation below
Explanation:
The molecular mass of sulfuric acid is
Number of moles is
Density of solution
molality
As-written, the question has no correct answer choice. Choice
The original question was:
The molality of 90% weight/volume H2SO4 solution is (density = 1.8 g/mL)
And we treat it as-written.
#90%"w/v" = ("90 g H"_2"SO"_4)/("100 mL solution")#
The solution is solute plus solvent, and mass is additive. Therefore:
#100 cancel"mL soln" xx "1.8 g"/cancel"mL" = "180 g solution"#
#= "90 g solute" + "90 g solvent"#
We said there was
#-> color(blue)("molality") = (90 cancel("g H"_2"SO"_4) xx "1 mol"/(98.079 cancel"g"))/(90 cancel"g solvent" xx "1 kg"/(1000 cancel"g"))#
#= "0.9176 mols solute"/"0.090 kg solvent"#
#=# #color(blue)("10.2 mol/kg")#
As a bonus, the molarity is...
#color(blue)("molarity") = "0.9176 mols solute"/"0.100 L soln"#
#=# #color(blue)("9.18 mols/L")#
In addition, the molarity of


