Please solve this phenol reaction based problem?
1 Answer
Mar 25, 2018
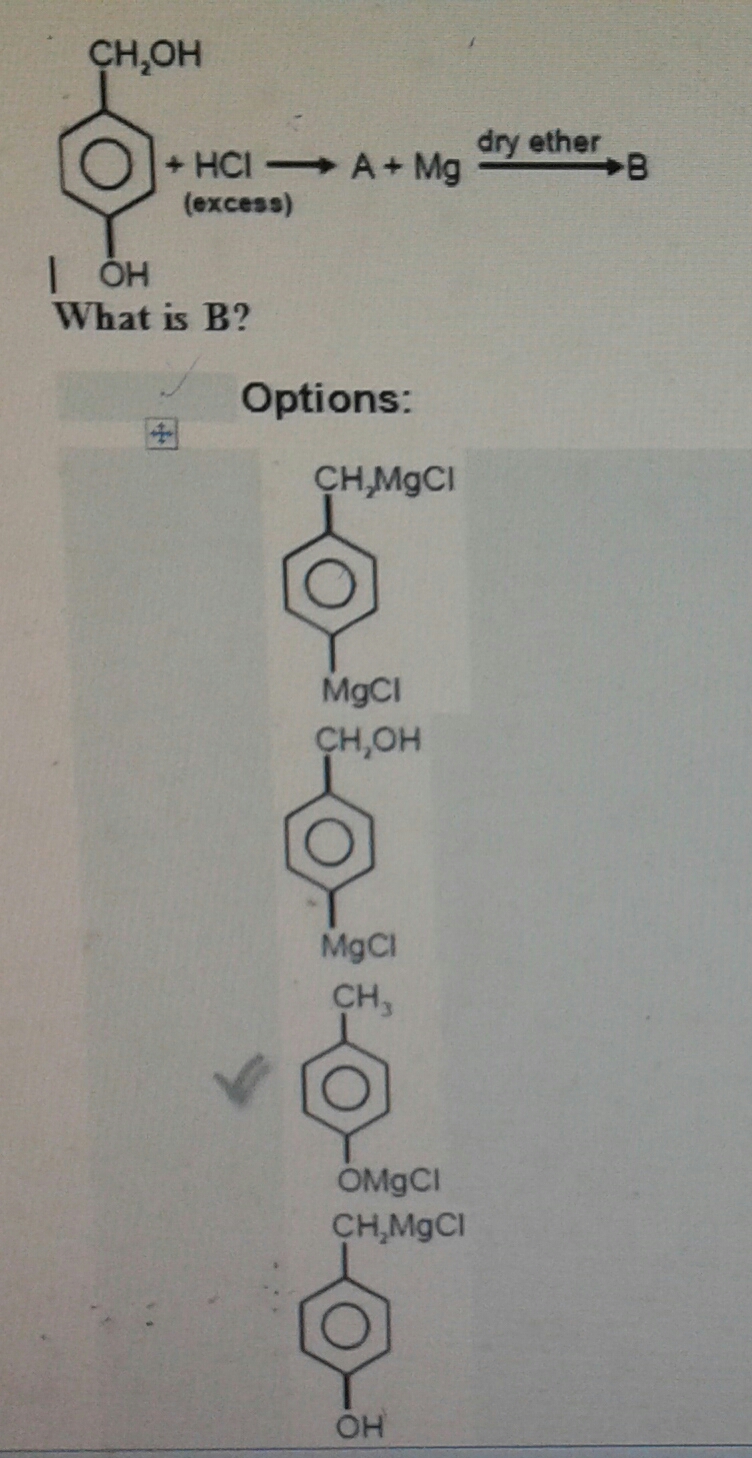
Well, it depends on how much excess... If we want to not waste money, then only the top
The
Then we just make the Grignard reagent... provided that the experiment is done properly. Water came off of the protonated alcohol, and must be dried off before reacting with

